Biphasic bisperoxovanadium administration and Schwann cell transplantation for repair after cervical contusive spinal cord injury
- PMID: 25510318
- PMCID: PMC4324167
- DOI: 10.1016/j.expneurol.2014.12.002
Biphasic bisperoxovanadium administration and Schwann cell transplantation for repair after cervical contusive spinal cord injury
Abstract
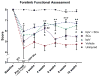
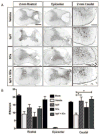
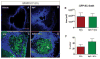
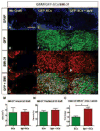
Schwann cells (SCs) hold promise for spinal cord injury (SCI) repair; however, there are limitations for its use as a lone treatment. We showed that acute inhibition of the phosphatase and tensin homolog deleted on chromosome ten (PTEN) by bisperoxovanadium (bpV) was neuroprotective and enhanced function following cervical hemicontusion SCI. We hypothesized that combining acute bpV therapy and delayed SC engraftment would further improve neuroprotection and recovery after cervical SCI. Adult female Sprague-Dawley (SD) rats were randomly sorted into 5 groups: sham, vehicle, bpV, SC transplantation, and bpV+SC transplantation. SCs were isolated from adult green fluorescent protein (GFP)-expressing SD rats (GFP-SCs). 200 μg/kg bpV(pic) was administered intraperitoneally (IP) twice daily for 7 days post-SCI in bpV-treated groups. GFP-SCs (1×10(6) in 5 μl medium) were transplanted into the lesion epicenter at the 8th day post-SCI. Forelimb function was tested for 10 weeks and histology was assessed. bpV alone significantly reduced lesion (by 40%, p<0.05) and cavitation (by 65%, p<0.05) and improved functional recovery (p<0.05) compared to injury alone. The combination promoted similar neuroprotection (p<0.01 vs. injury); however, GFP-SCs alone did not. Both SC-transplanted groups exhibited remarkable long-term SC survival, SMI-31(+) axon ingrowth and RECA-1(+) vasculature presence in the SC graft; however, bpV+SCs promoted an 89% greater axon-to-lesion ratio than SCs only. We concluded that bpV likely contributed largely to the neuroprotective and functional benefits while SCs facilitated considerable host-tissue interaction and modification. The combination of the two shows promise as an attractive strategy to enhance recovery after SCI.
Keywords: Neuroprotection; PTEN; Schwann cell transplantation; Spinal cord injury; bpV.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
PTEN inhibitor bisperoxovanadium protects oligodendrocytes and myelin and prevents neuronal atrophy in adult rats following cervical hemicontusive spinal cord injury.Neurosci Lett. 2014 Jun 24;573:64-8. doi: 10.1016/j.neulet.2014.02.039. Epub 2014 Feb 26. Neurosci Lett. 2014. PMID: 24582904 Free PMC article.
-
Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat.J Neurosci. 2015 Apr 29;35(17):6714-30. doi: 10.1523/JNEUROSCI.1070-14.2015. J Neurosci. 2015. PMID: 25926450 Free PMC article.
-
Long-term survival, axonal growth-promotion, and myelination of Schwann cells grafted into contused spinal cord in adult rats.Exp Neurol. 2014 Nov;261:308-19. doi: 10.1016/j.expneurol.2014.05.022. Epub 2014 May 27. Exp Neurol. 2014. PMID: 24873728 Free PMC article.
-
Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury.Handb Clin Neurol. 2012;109:523-40. doi: 10.1016/B978-0-444-52137-8.00032-2. Handb Clin Neurol. 2012. PMID: 23098734 Review.
-
Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus.Rev Neurosci. 2015;26(2):121-8. doi: 10.1515/revneuro-2014-0068. Rev Neurosci. 2015. PMID: 25581750 Review.
Cited by
-
Bisperoxovanadium protects against spinal cord injury by regulating autophagy via activation of ERK1/2 signaling.Drug Des Devel Ther. 2019 Feb 1;13:513-521. doi: 10.2147/DDDT.S187878. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 30774313 Free PMC article.
-
Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury.Stem Cells Transl Med. 2017 Oct;6(10):1917-1929. doi: 10.1002/sctm.17-0065. Epub 2017 Aug 22. Stem Cells Transl Med. 2017. PMID: 28834391 Free PMC article.
-
PTEN knockout using retrogradely transported AAVs transiently restores locomotor abilities in both acute and chronic spinal cord injury.Exp Neurol. 2023 Oct;368:114502. doi: 10.1016/j.expneurol.2023.114502. Epub 2023 Aug 8. Exp Neurol. 2023. PMID: 37558155 Free PMC article.
-
Acellularized spinal cord scaffolds incorporating bpV(pic)/PLGA microspheres promote axonal regeneration and functional recovery after spinal cord injury.RSC Adv. 2020 May 18;10(32):18677-18686. doi: 10.1039/d0ra02661a. eCollection 2020 May 14. RSC Adv. 2020. Retraction in: RSC Adv. 2023 Jan 16;13(4):2403. doi: 10.1039/d3ra90004e. PMID: 35518337 Free PMC article. Retracted.
-
PTEN knockout using retrogradely transported AAVs restores locomotor abilities in both acute and chronic spinal cord injury.bioRxiv [Preprint]. 2023 Apr 17:2023.04.17.537179. doi: 10.1101/2023.04.17.537179. bioRxiv. 2023. Update in: Exp Neurol. 2023 Oct;368:114502. doi: 10.1016/j.expneurol.2023.114502. PMID: 37131840 Free PMC article. Updated. Preprint.
References
-
- Barakat DJ, Gaglani SM, Neravetla SR, Sanchez AR, Andrade CM, Pressman Y, Puzis R, Garg MS, Bunge MB, Pearse DD. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell transplantation. 2005;14:225–240. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of neurotrauma. 1995;12:1–21. - PubMed
-
- Bresnahan JC, Beattie MS, Todd FDI, Noyes DH. A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Experimental neurology. 1987;95:548–570. - PubMed
-
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

