Tipping off endothelial tubes: nitric oxide drives tip cells
- PMID: 25510468
- PMCID: PMC5957492
- DOI: 10.1007/s10456-014-9455-0
Tipping off endothelial tubes: nitric oxide drives tip cells
Abstract
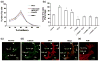
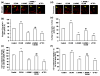
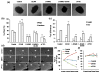
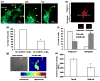
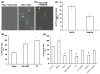
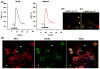
Angiogenesis, the formation of new blood vessels from pre-existing vessels, is a complex process that warrants cell migration, proliferation, tip cell formation, ring formation, and finally tube formation. Angiogenesis is initiated by a single leader endothelial cell called "tip cell," followed by vessel elongation by "stalk cells." Tip cells are characterized by their long filopodial extensions and expression of vascular endothelial growth factor receptor-2 and endocan. Although nitric oxide (NO) is an important modulator of angiogenesis, its role in angiogenic sprouting and specifically in tip cell formation is poorly understood. The present study tested the role of endothelial nitric oxide synthase (eNOS)/NO/cyclic GMP (cGMP) signaling in tip cell formation. In primary endothelial cell culture, about 40% of the tip cells showed characteristic sub-cellular localization of eNOS toward the anterior progressive end of the tip cells, and eNOS became phosphorylated at serine 1177. Loss of eNOS suppressed tip cell formation. Live cell NO imaging demonstrated approximately 35% more NO in tip cells compared with stalk cells. Tip cells showed increased level of cGMP relative to stalk cells. Further, the dissection of NO downstream signaling using pharmacological inhibitors and inducers indicates that NO uses the sGC/cGMP pathway in tip cells to lead angiogenesis. Taken together, the present study confirms that eNOS/NO/cGMP signaling defines the direction of tip cell migration and thereby initiates new blood vessel formation.
Figures



References
-
- Pyriochou A, Zhou Z, Koika V, Petrou C, Cordopatis P, Sessa WC, Papapetropoulos A. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J Cell Physiol. 2007;211:197–204. - PubMed
-
- Strasser GA, Kaminker JS, Tessier-Lavigne M. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 2010;115:5102–5110. - PubMed
-
- Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, Peltonen R, Freitas C, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

