Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence
- PMID: 25510500
- PMCID: PMC4288194
- DOI: 10.1093/nar/gku1304
Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence
Abstract
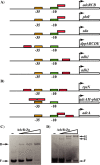
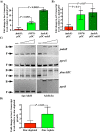
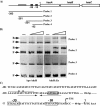
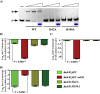
Altering zinc bioavailability to bacterial pathogens is a key component of host innate immunity. Thus, the ability to sense and adapt to the alterations in zinc concentrations is critical for bacterial survival and pathogenesis. To understand the adaptive responses of group A Streptococcus (GAS) to zinc limitation and its regulation by AdcR, we characterized gene regulation by AdcR. AdcR regulates the expression of 70 genes involved in zinc acquisition and virulence. Zinc-bound AdcR interacts with operator sequences in the negatively regulated promoters and mediates differential regulation of target genes in response to zinc deficiency. Genes involved in zinc mobilization and conservation are derepressed during mild zinc deficiency, whereas the energy-dependent zinc importers are upregulated during severe zinc deficiency. Further, we demonstrated that transcription activation by AdcR occurs by direct binding to the promoter. However, the repression and activation by AdcR is mediated by its interactions with two distinct operator sequences. Finally, mutational analysis of the metal ligands of AdcR caused impaired DNA binding and attenuated virulence, indicating that zinc sensing by AdcR is critical for GAS pathogenesis. Together, we demonstrate that AdcR regulates GAS adaptive responses to zinc limitation and identify molecular components required for GAS survival during zinc deficiency.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Andreini C., Banci L., Bertini I., Rosato A. Zinc through the three domains of life. J. Prot. Res. 2006;5:3173–3178. - PubMed
-
- Coleman J.E. Zinc enzymes. Curr. Opin. Chem. Biol. 1998;2:222–234. - PubMed
-
- Blencowe D.K., Morby A.P. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003;27:291–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

