Tuning reactivity and site selectivity of simple arenes in C-H activation: ortho-arylation of anisoles via arene-metal π-complexation
- PMID: 25510851
- PMCID: PMC4379957
- DOI: 10.1021/ja510260j
Tuning reactivity and site selectivity of simple arenes in C-H activation: ortho-arylation of anisoles via arene-metal π-complexation
Abstract
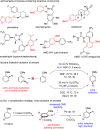
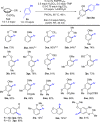
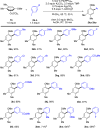
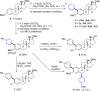
Current approaches to achieve site selectivity in the C-H activation of arenes involve the use of directing groups or highly electron-poor arenes. In contrast, simple arenes, such as anisole, are characterized by poor reactivity and selectivity. We report that π-complexation to a Cr(CO)3 unit enhances the reactivity of anisoles providing an unprecedented ortho-selective arylation. This mild methodology can be used for the late stage functionalization of bioactive compounds containing the anisole motif, allowing the construction of novel organic scaffolds with few synthetic steps.
Figures
References
-
-
For recent reviews on direct arylation reactions and Pd-catalyzed C–H activation methodologies, see:
- Alberico D.; Scott M. E.; Lautens M. Chem. Rev. 2007, 107, 174. - PubMed
- Ackermann L.; Vicente R.; Kapdi A. R. Angew. Chem., Int. Ed. 2009, 48, 9792. - PubMed
- Chen X.; Engle K. M.; Wang D.-H.; Yu J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094. - PMC - PubMed
- Daugulis O.; Do H.-Q.; Shabashov D. Acc. Chem. Res. 2009, 42, 1074. - PMC - PubMed
- McGlacken G. P.; Bateman L. M. Chem. Soc. Rev. 2009, 38, 2447. - PubMed
- Ashenhurst J. A. Chem. Soc. Rev. 2010, 39, 540. - PubMed
- Lyons T. W.; Sanford M. S. Chem. Rev. 2010, 110, 1147. - PMC - PubMed
- Yu J.-Q., Shi Z., Eds. Topics in Current Chemistry: C–H Activation, 1st ed.; Springer: Berlin/Heidelberg, 2010.
- Wencel-Delord J.; Dröge T.; Liu F.; Glorius F. Chem. Soc. Rev. 2011, 40, 4740. - PubMed
- Hussain I.; Singh T. Adv. Synth. Catal. 2014, 356, 1661. - PMC - PubMed
-
-
- Chiong H. A.; Pham Q.-N.; Daugulis O. J. Am. Chem. Soc. 2007, 129, 9879. - PubMed
- Wang D.-H.; Mei T.-S.; Yu J.-Q. J. Am. Chem. Soc. 2008, 130, 17676. - PMC - PubMed
- Nishikata T.; Abela A. R.; Huang S.; Lipshutz B. H. J. Am. Chem. Soc. 2010, 132, 4978. - PMC - PubMed
- Cornella J.; Righi M.; Larrosa I. Angew. Chem., Int. Ed. 2011, 50, 9429. - PubMed
- Tredwell M. J.; Gulias M.; Bremeyer N. G.; Johansson C. C. C.; Collins B. S. L.; Gaunt M. J. Angew. Chem., Int. Ed. 2011, 50, 1076. - PubMed
- Kalyani D.; McMurtrey K. B.; Neufeldt S. R.; Sanford M. S. J. Am. Chem. Soc. 2011, 133, 18566. - PMC - PubMed
- Ackermann L.; Pospech J.; Potukuchi H. K. Org. Lett. 2012, 14, 2146. - PubMed
- Gulevich A. V.; Melkonyan F. S.; Sarkar D.; Gevorgyan V. J. Am. Chem. Soc. 2012, 134, 5528. - PMC - PubMed
- Pan F.; Lei Z.-Q.; Wang H.; Li H.; Sun J.; Shi Z.-J. Angew. Chem., Int. Ed. 2013, 52, 2063. - PubMed
- Aihara Y.; Chatani N. Chem. Sci. 2013, 4, 664.
- Arroniz C.; Ironmonger A.; Rassias G.; Larrosa I. Org. Lett. 2013, 15, 910. - PubMed
- Arroniz C.; Denis J. G.; Ironmonger A.; Rassias G.; Larrosa I. Chem. Sci. 2014, 5, 3509.
-
-
For examples of meta-arylation:
- Phipps R. J.; Gaunt M. J. Science 2009, 323, 1593. - PubMed
- Duong H. A.; Gilligan R. E.; Cooke M. L.; Phipps R. J.; Gaunt M. J. Angew. Chem., Int. Ed. 2011, 50, 463. - PubMed
- Wan L.; Dastbaravardeh N.; Li G.; Yu J.-Q. J. Am. Chem. Soc. 2013, 135, 18056. - PMC - PubMed
- Luo J.; Preciado S.; Larrosa I. J. Am. Chem. Soc. 2014, 136, 4109. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

