Developing a xylanase XYNZG from Plectosphaerella cucumerina for baking by heterologously expressed in Kluyveromyces lactis
- PMID: 25511290
- PMCID: PMC4297440
- DOI: 10.1186/s12896-014-0107-7
Developing a xylanase XYNZG from Plectosphaerella cucumerina for baking by heterologously expressed in Kluyveromyces lactis
Abstract
Background: Xylanase can replace chemical additives to improve the volume and sensory properties of bread in the baking. Suitable baking xylanase with improved yield will promote the application of xylanase in baking industry. The xylanase XYNZG from the Plectosphaerella cucumerina has been previously characterized by heterologous expression in Pichia pastoris. However, P. pastoris is not a suitable host for xylanase to be used in the baking process since P. pastoris does not have GRAS (Generally Regarded As Safe) status and requires large methanol supplement during the fermentation in most conditions, which is not allowed to be used in the food industry. Kluyveromyces lactis, as another yeast expression host, has a GRAS status, which has been successfully used in food and feed applications. No previous work has been reported concerning the heterologous expression of xylanase gene xynZG in K. lactis with an aim for application in baking.
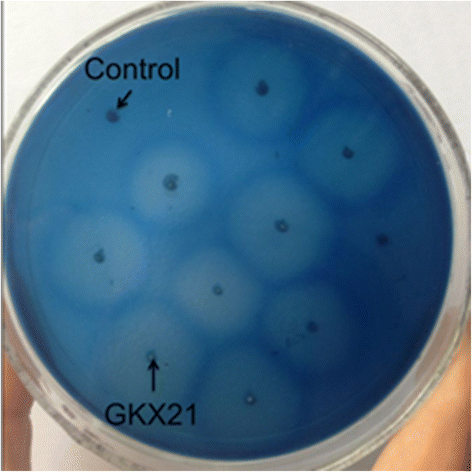
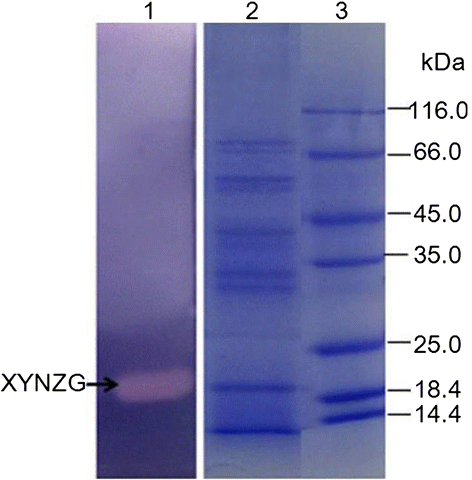
Results: The xylanase gene xynZG from the P. cucumerina was heterologously expressed in K. lactis. The recombinant protein XYNZG in K. lactis presented an approximately 19 kDa band on SDS-PAGE and zymograms analysis. Transformant with the highest halo on the plate containing the RBB-xylan (Remazol Brilliant Blue-xylan) was selected for the flask fermentation in different media. The results indicated that the highest activity of 115 U/ml at 72 h was obtained with the YLPU medium. The mass spectrometry analysis suggested that the hydrolytic products of xylan by XYNZG were mainly xylobiose and xylotriose. The results of baking trials indicated that the addition of XYNZG could reduce the kneading time of dough, increase the volume of bread, improve the texture, and have more positive effects on the sensory properties of bread.
Conclusions: Xylanase XYNZG is successfully expressed in K. lactis, which exhibits the highest activity among the published reports of the xylanase expression in K. lactis. The recombinant XYNZG can be used to improve the volume and sensory properties of bread. Therefore, the expression yield of recombinant XYNZG can be further improved through engineered strain containing high copy numbers of the XYNZG, and optimized fermentation condition, making bread-baking application possible.
Figures




References
-
- Collins T, Hoyoux A, Dutron A, Georis J, Genot B, Dauvrin T, Arnaut F, Gerday C, Feller G. Use of glycoside hydrolase family 8 xylanases in baking. J Cereal Sci. 2006;43(1):79–84. doi: 10.1016/j.jcs.2005.08.002. - DOI
-
- Courtin C, Delcour JA. Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci. 2002;35(3):225–243. doi: 10.1006/jcrs.2001.0433. - DOI
-
- Shrivastava S, Shukla P, Deepalakshmi PD, Mukhopadhyay K. Characterization, cloning and functional expression of novel xylanase from Thermomyces lanuginosus SS-8 isolated from self-heating plant wreckage material. World J Microbiol Biotechnol. 2013;29(12):2407–2415. doi: 10.1007/s11274-013-1409-y. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

