Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells
- PMID: 25512109
- PMCID: PMC4301465
- DOI: 10.1186/1471-2407-14-955
Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells
Abstract
Background: Cancer-associated fibroblasts and high mobility group box 1 (HMGB1) protein have been suggested to mediate cancer progression and chemotherapy resistance. The role of such fibroblasts in HMGB1 production in breast cancer is unclear. This study aimed to investigate the effects of cancer-associated fibroblasts on HMGB1 expression in breast cancer cells and its role in chemotherapeutic response.
Methods: Breast cancer-associated fibroblasts (BCFs) and non-tumor-associated fibroblasts (NTFs) were isolated from human breast cancers or adjacent normal tissues and established as primary cultures in vitro. After confirmation of the activated status of these fibroblasts, conditioned-media (CM) were collected and applied to MDA-MB-231 human triple negative breast cancer cells. The levels of intracellular and extracellular HMGB1 were measured by real-time PCR and/or Western blot. The response of BCF-CM-pre-treated cancer cells to doxorubicin (Dox) was compared with those pre-treated with NTF-CM or control cultures. The effect of an HMGB1 neutralizing antibody on Dox resistance induced by extracellular HMGB1 from non-viable Dox-treated cancer cells or recombinant HMGB1 was also investigated.
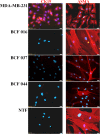
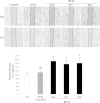
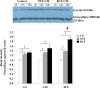
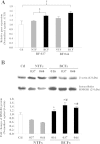
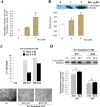
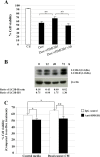
Results: Immunocytochemical analysis revealed that BCFs and NTFs were alpha-smooth muscle actin (ASMA) positive and cytokeratin 19 (CK19) negative cells: a phenotype consistent with that of activated fibroblasts. We confirmed that the CM from BCFs (but not NTFs), could significantly induce breast cancer cell migration. Intracellular HMGB1 expression was induced in BCF-CM-treated breast cancer cells and also in Dox-treated cells. Extracellular HMGB1 was strongly expressed in the CM after Dox-induced MDA-MB-231 cell death and was higher in cells pre-treated with BCF-CM than NTF-CM. Pre-treatment of breast cancer cells with BCF-CM induced a degree of resistance to Dox in accordance with the increased level of secreted HMGB1. Recombinant HMGB1 was shown to increase Dox resistance and this was associated with evidence of autophagy. Anti-HMGB1 neutralizing antibody significantly reduced the effect of extracellular HMGB1 released from dying cancer cells or of recombinant HMGB1 on Dox resistance.
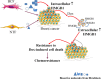
Conclusions: These findings highlight the potential of stromal fibroblasts to contribute to chemoresistance in breast cancer cells in part through fibroblast-induced HMGB1 production.
Figures
References
-
- Attasara P, Buasom R. Hospital-based cancer registry 2010. Natl Cancer Inst Thai. 2010;24:1–52.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/955/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

