Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects
- PMID: 25512407
- PMCID: PMC4335848
- DOI: 10.1128/AAC.04223-14
Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects
Erratum in
-
Erratum for Kersemaekers et al., Pharmacokinetics and Safety Study of Posaconazole Intravenous Solution Administered Peripherally to Healthy Subjects.Antimicrob Agents Chemother. 2016 Jun 20;60(7):4426. doi: 10.1128/AAC.01001-16. Print 2016 Jul. Antimicrob Agents Chemother. 2016. PMID: 27325815 Free PMC article. No abstract available.
Abstract
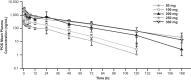
This study evaluated the safety, tolerability, and pharmacokinetics of a posaconazole i.v. (intravenous) solution. This was a single-center, 2-part, randomized, rising single- and multiple-dose study in healthy adults. In part 1, subjects received 0 (vehicle), 50, 100, 200, 250, or 300 mg posaconazole in a single dose i.v. by 30-min peripheral infusion (6 cohorts of 12 subjects each [9 active and 3 placebo], making a total of 72 subjects). Blood samples were collected until 168 h postdose. In part 2, subjects were to receive 2 peripheral infusions at a 12-h interval on day 1 followed by once-daily infusion for 9 days. However, part 2 was terminated early because of high rates of infusion site reactions with multiple dosing at the same infusion site. The pharmacokinetics results for part 1 (n=45 subjects) showed that the mean posaconazole exposure (area under the concentration-time curve from time zero to infinity [AUC0-∞]) ranged from 4,890 to 46,400 ng · h/ml (range of coefficient of variation values, 26 to 50). The dose-proportionality slope estimate (90% confidence interval) for AUC0-∞ was 1.30 (1.19 to 1.41), indicating a greater-than-dose-proportional increase. The data for safety in part 1 show that 29/72 subjects had ≥1 adverse event. Infusion site reactions were reported in 2/9 vehicle subjects, 0/18 placebo subjects, and 7/45 i.v. posaconazole subjects. The data for safety in part 2 show that infusion site reactions were reported in 1/4 (25%) placebo subjects, 3/9 (33%) vehicle control subjects, and 4/5 (80%) i.v. posaconazole (100 mg) subjects (3 posaconazole recipients subsequently developed thrombophlebitis and were discontinued from treatment). In conclusion, the posaconazole i.v. solution showed a greater-than-dose-proportional increase in exposure, primarily at doses below 200 mg. When administered peripherally at the same infusion site, multiple dosing of i.v. posaconazole led to unacceptably high rates of infusion site reactions. Intravenous posaconazole was otherwise well tolerated. Single doses of i.v. posaconazole were tolerated when given through a peripheral vein over 30 min.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi:10.1056/NEJMoa061094. - DOI - PubMed
-
- Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356:335–347. doi:10.1056/NEJMoa061098. - DOI - PubMed
-
- Keating GM. 2005. Posaconazole. Drugs 65:1553–1567. - PubMed
-
- Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 44:2–12. doi:10.1086/508774. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

