Randomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients With Advanced Pancreatic Cancer
- PMID: 25512461
- PMCID: PMC4881365
- DOI: 10.1200/JCO.2014.55.7504
Randomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients With Advanced Pancreatic Cancer
Abstract
Purpose: TH-302 is an investigational hypoxia-activated prodrug that releases the DNA alkylator bromo-isophosphoramide mustard in hypoxic settings. This phase II study (NCT01144455) evaluated gemcitabine plus TH-302 in patients with previously untreated, locally advanced or metastatic pancreatic cancer.
Patients and methods: Patients were randomly assigned 1:1:1 to gemcitabine (1,000 mg/m(2)), gemcitabine plus TH-302 240 mg/m(2) (G+T240), or gemcitabine plus TH-302 340 mg/m(2) (G+T340). Randomized crossover after progression on gemcitabine was allowed. The primary end point was progression-free survival (PFS). Secondary end points included overall survival (OS), tumor response, CA 19-9 response, and safety.
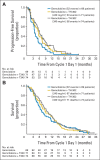
Results: Two hundred fourteen patients (77% with metastatic disease) were enrolled between June 2010 and July 2011. PFS was significantly longer with gemcitabine plus TH-302 (pooled combination arms) compared with gemcitabine alone (median PFS, 5.6 v 3.6 months, respectively; hazard ratio, 0.61; 95% CI, 0.43 to 0.87; P = .005; median PFS for metastatic disease, 5.1 v 3.4 months, respectively). Median PFS times for G+T240 and G+T340 were 5.6 and 6.0 months, respectively. Tumor response was 12%, 17%, and 26% in the gemcitabine, G+T240, and G+T340 arms, respectively (G+T340 v gemcitabine, P = .04). CA 19-9 decrease was greater with G+T340 versus gemcitabine (-5,398 v -549 U/mL, respectively; P = .008). Median OS times for gemcitabine, G+T240, and G+T340 were 6.9, 8.7, and 9.2 months, respectively (P = not significant). The most common adverse events (AEs) were fatigue, nausea, and peripheral edema (frequencies similar across arms). Skin and mucosal toxicities (2% grade 3) and myelosuppression (55% grade 3 or 4) were the most common TH-302-related AEs but were not associated with treatment discontinuation.
Conclusion: PFS, tumor response, and CA 19-9 response were significantly improved with G+TH-302. G+T340 is being investigated further in the phase III MAESTRO study (NCT01746979).
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Targeting tumor hypoxia with hypoxia-activated prodrugs.J Clin Oncol. 2015 May 1;33(13):1505-8. doi: 10.1200/JCO.2014.60.0759. Epub 2015 Mar 23. J Clin Oncol. 2015. PMID: 25800764 No abstract available.
-
[TH-302: a new hypoxia-activated cytostatic agent in pancreatic cancer treatment].Strahlenther Onkol. 2015 Oct;191(10):819-20. doi: 10.1007/s00066-015-0877-4. Strahlenther Onkol. 2015. PMID: 26385861 German. No abstract available.
References
-
- Royal RE, Wolfe RA, Crane CH. Cancers of the pancreas. In: Devita VT Jr, Lawrence TS, Rosenberg SA, editors. Principles and Practice of Oncology (ed 9) Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 961–989.
-
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical