Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity
- PMID: 25512469
- PMCID: PMC4291534
- DOI: 10.1084/jem.20130062
Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity
Abstract
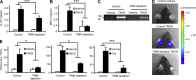
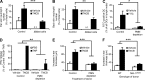
Allergic contact dermatitis and its animal model, contact hypersensitivity (CHS), are T cell-mediated inflammatory skin diseases induced by contact allergens. Though numerous cellular and molecular players are known, the mechanism of chemical-induced sensitization remains poorly understood. Here, we identify neutrophils as crucial players in the sensitization phase of CHS. Genetic deficiency of neutrophils caused by myeloid-specific deletion of Mcl-1 or antibody-mediated depletion of neutrophils before sensitization abrogated the CHS response. Neutrophil deficiency reduced contact allergen-induced cytokine production, gelatinase release, and reactive oxygen species production in naive mice. Mast cell deficiency inhibited neutrophil accumulation at the site of sensitization. In turn, neutrophils were required for contact allergen-induced release of further neutrophil-attracting chemokines, migration of DCs to the draining lymph nodes, and priming of allergen-specific T cells. Lymph node cells from mice sensitized in the absence of neutrophils failed to transfer sensitization to naive recipients. Furthermore, no CHS response could be induced when neutrophils were depleted before elicitation or when normally sensitized lymph node cells were transferred to neutrophil-deficient recipients, indicating an additional role for neutrophils in the elicitation phase. Collectively, our data identify neutrophils to be critically involved in both the sensitization and elicitation phase of CHS.
© 2015 Weber et al.
Figures
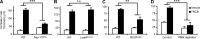
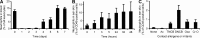
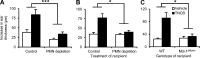
References
-
- Charmoy M., Milon G., and Tacchini-Cottier F.. 2011. Role of Neutrophils in the Early Shaping of the Leishmania major Specific Immune Response in Experimental Murine Cutaneous Leishmaniasis. Neutrophils in Infectious Diseases. Tacchini-Cottier F., and van Zandbergen G., Bentham Science Publishers Ltd. pp. 49–58.
-
- Cumberbatch M., Dearman R.J., Groves R.W., Antonopoulos C., and Kimber I.. 2002. Differential regulation of epidermal langerhans cell migration by interleukins (IL)-1alpha and IL-1beta during irritant- and allergen-induced cutaneous immune responses. Toxicol. Appl. Pharmacol. 182:126–135 10.1006/taap.2002.9442 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

