Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma
- PMID: 25512523
- PMCID: PMC4280630
- DOI: 10.1073/pnas.1419260111
Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma
Abstract
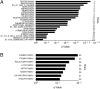
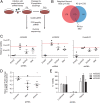
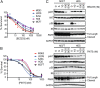
Osteosarcoma is the most common primary bone tumor, yet there have been no substantial advances in treatment or survival in three decades. We examined 59 tumor/normal pairs by whole-exome, whole-genome, and RNA-sequencing. Only the TP53 gene was mutated at significant frequency across all samples. The mean nonsilent somatic mutation rate was 1.2 mutations per megabase, and there was a median of 230 somatic rearrangements per tumor. Complex chains of rearrangements and localized hypermutation were detected in almost all cases. Given the intertumor heterogeneity, the extent of genomic instability, and the difficulty in acquiring a large sample size in a rare tumor, we used several methods to identify genomic events contributing to osteosarcoma survival. Pathway analysis, a heuristic analytic algorithm, a comparative oncology approach, and an shRNA screen converged on the phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway as a central vulnerability for therapeutic exploitation in osteosarcoma. Osteosarcoma cell lines are responsive to pharmacologic and genetic inhibition of the PI3K/mTOR pathway both in vitro and in vivo.
Keywords: PI3K; TP53; genomics; mTOR; osteosarcoma.
Conflict of interest statement
Conflict of interest statement: C.W.M.R. receives research support and consulting fees from the Novartis Institutes for Biomedical Research (NIBR) via the Dana–Farber Cancer Institute/NIBR drug discovery program.
Figures


Comment in
-
Targeting osteosarcoma.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18100-1. doi: 10.1073/pnas.1420596111. Epub 2014 Dec 15. Proc Natl Acad Sci U S A. 2014. PMID: 25512494 Free PMC article. No abstract available.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- McIntyre JF, et al. Germline mutations of the p53 tumor suppressor gene in children with osteosarcoma. J Clin Oncol. 1994;12(5):925–930. - PubMed
-
- Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26(1):1–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

