Structural model of the open-closed-inactivated cycle of prokaryotic voltage-gated sodium channels
- PMID: 25512599
- PMCID: PMC4278185
- DOI: 10.1085/jgp.201411242
Structural model of the open-closed-inactivated cycle of prokaryotic voltage-gated sodium channels
Abstract
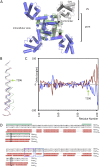
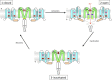
In excitable cells, the initiation of the action potential results from the opening of voltage-gated sodium channels. These channels undergo a series of conformational changes between open, closed, and inactivated states. Many models have been proposed for the structural transitions that result in these different functional states. Here, we compare the crystal structures of prokaryotic sodium channels captured in the different conformational forms and use them as the basis for examining molecular models for the activation, slow inactivation, and recovery processes. We compare structural similarities and differences in the pore domains, specifically in the transmembrane helices, the constrictions within the pore cavity, the activation gate at the cytoplasmic end of the last transmembrane helix, the C-terminal domain, and the selectivity filter. We discuss the observed differences in the context of previous models for opening, closing, and inactivation, and present a new structure-based model for the functional transitions. Our proposed prokaryotic channel activation mechanism is then compared with the activation transition in eukaryotic sodium channels.
© 2015 Bagnéris et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

