Glycolysis controls plasma membrane glucose sensors to promote glucose signaling in yeasts
- PMID: 25512610
- PMCID: PMC4301714
- DOI: 10.1128/MCB.00515-14
Glycolysis controls plasma membrane glucose sensors to promote glucose signaling in yeasts
Abstract
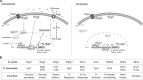
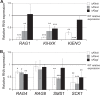
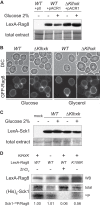
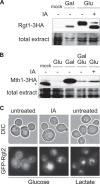
Sensing of extracellular glucose is necessary for cells to adapt to glucose variation in their environment. In the respiratory yeast Kluyveromyces lactis, extracellular glucose controls the expression of major glucose permease gene RAG1 through a cascade similar to the Saccharomyces cerevisiae Snf3/Rgt2/Rgt1 glucose signaling pathway. This regulation depends also on intracellular glucose metabolism since we previously showed that glucose induction of the RAG1 gene is abolished in glycolytic mutants. Here we show that glycolysis regulates RAG1 expression through the K. lactis Rgt1 (KlRgt1) glucose signaling pathway by targeting the localization and probably the stability of Rag4, the single Snf3/Rgt2-type glucose sensor of K. lactis. Additionally, the control exerted by glycolysis on glucose signaling seems to be conserved in S. cerevisiae. This retrocontrol might prevent yeasts from unnecessary glucose transport and intracellular glucose accumulation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Wésolowski-Louvel M, Goffrini P, Ferrero I, Fukuhara H. 1992. Glucose transport in the yeast Kluyveromyces lactis. I. Properties of an inducible low-affinity glucose transporter gene. Mol Gen Genet 233:89–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
