MRI assessment of early response to certolizumab pegol in rheumatoid arthritis: a randomised, double-blind, placebo-controlled phase IIIb study applying MRI at weeks 0, 1, 2, 4, 8 and 16
- PMID: 25512675
- PMCID: PMC4431335
- DOI: 10.1136/annrheumdis-2014-206359
MRI assessment of early response to certolizumab pegol in rheumatoid arthritis: a randomised, double-blind, placebo-controlled phase IIIb study applying MRI at weeks 0, 1, 2, 4, 8 and 16
Abstract
Objectives: To identify the first time point of an MRI-verified response to certolizumab pegol (CZP) therapy in patients with rheumatoid arthritis (RA).
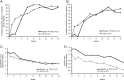
Methods: Forty-one patients with active RA despite disease-modifying antirheumatic drug therapy were randomised 2:1 to CZP (CZP loading dose 400 mg every 2 weeks at weeks 0-4; CZP 200 mg every 2 weeks at weeks 6-16) or placebo→CZP (placebo at weeks 0-2; CZP loading dose at weeks 2-6; CZP 200 mg every 2 weeks at weeks 8-16). Contrast-enhanced MRI of one hand and wrist was acquired at baseline (week 0) and weeks 1, 2, 4, 8 and 16. All six time points were read simultaneously, blinded to time, using the Outcome Measures in Rheumatology Clinical Trials RA MRI scoring system. Primary outcome was change in synovitis score in the CZP group; secondary outcomes were change in bone oedema (osteitis) and erosion scores and clinical outcome measures.
Results: Forty patients were treated (27 CZP, 13 placebo→CZP), and 36 (24 CZP, 12 placebo→CZP) completed week 16. In the CZP group, there were significant reductions from baseline synovitis (Hodges-Lehmann estimate of median change, -1.5, p=0.049) and osteitis scores (-2.5, p=0.031) at week 16. Numerical, but statistically insignificant, MRI inflammation reductions were observed at weeks 1-2 in the CZP group. No significant change was seen in bone erosion score. Improvements across all clinical outcomes were seen in the CZP group.
Conclusions: CZP reduced MRI synovitis and osteitis scores at week 16, despite small sample size and the technical challenge of reading six time points simultaneously. This study provides essential information on optimal MRI timing for subsequent trials.
Trial registration number: ClinicalTrials.gov, NCT01235598.
Keywords: Anti-TNF; Inflammation; Magnetic Resonance Imaging; Rheumatoid Arthritis; Synovitis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
-
- Aletaha D, Smolen J, Ward MM. Measuring function in rheumatoid arthritis: Identifying reversible and irreversible components. Arthritis Rheum 2006;54:2784–92. - PubMed
-
- Boyesen P, Haavardsholm EA, Østergaard M, et al. . MRI in early rheumatoid arthritis: synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann Rheum Dis 2011;70:428–33. - PubMed
-
- Hetland ML, Ejbjerg B, Horslev-Petersen K, et al. . MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis 2009;68:384–90. - PubMed
-
- Van Leeuwen M, Van der Heijde D, Van Rijswijk M, et al. . Interrelationship of outcome measures and process variables in early rheumatoid arthritis. A comparison of radiologic damage, physical disability, joint counts, and acute phase reactants. J Rheumatol 1994;21:425–9. - PubMed
-
- Haavardsholm EA, Østergaard M, Ejbjerg BJ, et al. . Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum 2005;52:3860–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

