Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial
- PMID: 25513807
- PMCID: PMC4267810
- DOI: 10.1371/journal.pmed.1001768
Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial
Abstract
Background: The success of HIV programs relies on widely accessible HIV testing and counseling (HTC) services at health facilities as well as in the community. Home-based HTC (HB-HTC) is a popular community-based approach to reach persons who do not test at health facilities. Data comparing HB-HTC to other community-based HTC approaches are very limited. This trial compares HB-HTC to mobile clinic HTC (MC-HTC).
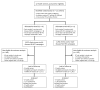
Methods and findings: The trial was powered to test the hypothesis of higher HTC uptake in HB-HTC campaigns than in MC-HTC campaigns. Twelve clusters were randomly allocated to HB-HTC or MC-HTC. The six clusters in the HB-HTC group received 30 1-d multi-disease campaigns (five villages per cluster) that delivered services by going door-to-door, whereas the six clusters in MC-HTC group received campaigns involving community gatherings in the 30 villages with subsequent service provision in mobile clinics. Time allocation and human resources were standardized and equal in both groups. All individuals accessing the campaigns with unknown HIV status or whose last HIV test was >12 wk ago and was negative were eligible. All outcomes were assessed at the individual level. Statistical analysis used multivariable logistic regression. Odds ratios and p-values were adjusted for gender, age, and cluster effect. Out of 3,197 participants from the 12 clusters, 2,563 (80.2%) were eligible (HB-HTC: 1,171; MC-HTC: 1,392). The results for the primary outcomes were as follows. Overall HTC uptake was higher in the HB-HTC group than in the MC-HTC group (92.5% versus 86.7%; adjusted odds ratio [aOR]: 2.06; 95% CI: 1.18-3.60; p = 0. 011). Among adolescents and adults ≥ 12 y, HTC uptake did not differ significantly between the two groups; however, in children <12 y, HTC uptake was higher in the HB-HTC arm (87.5% versus 58.7%; aOR: 4.91; 95% CI: 2.41-10.0; p<0.001). Out of those who took up HTC, 114 (4.9%) tested HIV-positive, 39 (3.6%) in the HB-HTC arm and 75 (6.2%) in the MC-HTC arm (aOR: 0.64; 95% CI: 0.48-0.86; p = 0.002). Ten (25.6%) and 19 (25.3%) individuals in the HB-HTC and in the MC-HTC arms, respectively, linked to HIV care within 1 mo after testing positive. Findings for secondary outcomes were as follows: HB-HTC reached more first-time testers, particularly among adolescents and young adults, and had a higher proportion of men among participants. However, after adjusting for clustering, the difference in male participation was not significant anymore. Age distribution among participants and immunological and clinical stages among persons newly diagnosed HIV-positive did not differ significantly between the two groups. Major study limitations included the campaigns' restriction to weekdays and a relatively low HIV prevalence among participants, the latter indicating that both arms may have reached an underexposed population.
Conclusions: This study demonstrates that both HB-HTC and MC-HTC can achieve high uptake of HTC. The choice between these two community-based strategies will depend on the objective of the activity: HB-HTC was better in reaching children, individuals who had never tested before, and men, while MC-HTC detected more new HIV infections. The low rate of linkage to care after a positive HIV test warrants future consideration of combining community-based HTC approaches with strategies to improve linkage to care for persons who test HIV-positive.
Trial registration: ClinicalTrials.gov NCT01459120. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS (2010) Getting to zero: 2011–2015 strategy. Geneva: Joint United Nations Programme on HIV/AIDS.
-
- Joint United Nations Programme on HIV/AIDS (2012) UNAIDS report on the global AIDS epidemic 2012. Geneva: Joint United Nations Programme on HIV/AIDS.
-
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG (2009) Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373: 48–57. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical