Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4
- PMID: 25514209
- PMCID: PMC4267812
- DOI: 10.1371/journal.pone.0115210
Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4
Abstract
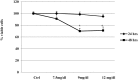
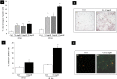
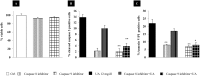
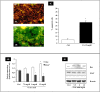
Mild hyperuricemia has been linked to the development and progression of tubulointerstitial renal damage. However the mechanisms by which uric acid may cause these effects are poorly explored. We investigated the effect of uric acid on apoptosis and the underlying mechanisms in a human proximal tubule cell line (HK-2). Increased uric acid concentration decreased tubule cell viability and increased apoptotic cells in a dose dependent manner (up to a 7-fold increase, p<0.0001). Uric acid up-regulated Bax (+60% with respect to Ctrl; p<0.05) and down regulated X-linked inhibitor of apoptosis protein. Apoptosis was blunted by Caspase-9 but not Caspase-8 inhibition. Uric acid induced changes in the mitochondrial membrane, elevations in reactive oxygen species and a pronounced up-regulation of NOX 4 mRNA and protein (p<0.05). In addition, both reactive oxygen species production and apoptosis was prevented by the NADPH oxidase inhibitor DPI as well as by Nox 4 knockdown. URAT 1 transport inhibition by probenecid and losartan and its knock down by specific siRNA, blunted apoptosis, suggesting a URAT 1 dependent cell death. In summary, our data show that uric acid increases the permissiveness of proximal tubule kidney cells to apoptosis by triggering a pathway involving NADPH oxidase signalling and URAT 1 transport. These results might explain the chronic tubulointerstitial damage observed in hyperuricaemic states and suggest that uric acid transport in tubular cells is necessary for urate-induced effects.
Conflict of interest statement
Figures




References
-
- Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, et al. (2001) Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res 24:691–697. - PubMed
-
- Viazzi F, Leoncini G, Ratto E, Falqui V, Parodi A, et al. (2007) Mild hyperuricemia and subclinical renal damage in untreated primary hypertension. Am J Hypertens 20:1276–1282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

