Early developability screen of therapeutic antibody candidates using Taylor dispersion analysis and UV area imaging detection
- PMID: 25514497
- PMCID: PMC4623059
- DOI: 10.4161/19420862.2014.985544
Early developability screen of therapeutic antibody candidates using Taylor dispersion analysis and UV area imaging detection
Abstract
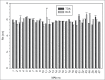
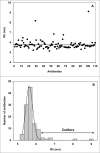
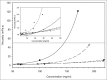
Therapeutic antibodies represent one of the fastest growing segments in the pharmaceutical market. They are used in a broad range of disease fields, such as autoimmune diseases, cancer, inflammation and infectious diseases. The growth of the segment has necessitated development of new analytical platforms for faster and better antibody selection and characterization. Early quality control and risk assessment of biophysical parameters help prevent failure in later stages of antibody development, and thus can reduce costs and save time. Critical parameters such as aggregation, conformational stability, colloidal stability and hydrophilicity, are measured during the early phase of antibody generation and guide the selection process of the best lead candidates in terms of technical developability. We report on the use of a novel instrument (ActiPix/Viscosizer) for measuring both the hydrodynamic radius and the absolute viscosity of antibodies based on Taylor dispersion analysis and UV area imaging. The looped microcapillary-based method combines low sample consumption, fast throughput and high precision compared to other conventional methods. From a random panel of 130 antibodies in the early selection process, we identified some with large hydrodynamic radius outside the normal distribution and others with non-Gaussian Taylor dispersion profiles. The antibodies with such abnormal properties were confirmed later in the selection process to show poor developability profiles. Moreover, combining these results with those of the viscosity measurements at high antibody concentrations allows screening, with limited amounts of materials, candidates with potential issues in pre-formulation development.
Keywords: APS; Dulbecco phosphate buffered saline; hIgG; Taylor dispersion; Taylor dispersion analysis; CE; UV array imaging detection; active pixel sensor; TDA; capillary electrophoresis; DLS; coefficient of variation; developability assessment; dynamic light scattering; dPBS; fragment antigen-binding; ID; human immunoglobulin G; mIgG; hydrodynamic radius; hydrodynamic radius; S-DAS; internal diameter; OD; millipascal seconds; Rh; monoclonal antibodies; monoclonal antibody; Fab; mouse immunoglobulin G; mAb; outside diameter; mPa.s; scaling factor; STD; selection developability assessment; SEC-LC; size-exclusion liquid chromatography; SF; standard deviation; CV; viscosity.
Figures




References
-
- Reichert JM. Antibodies to watch in 2014. MAbs 2014; 6:5-14; PMID:24284914; http://dx.doi.org/10.4161/mabs.27333 - DOI - PMC - PubMed
-
- Yang X, Xu W, Dukleska S, Benchaar S, Mengisen S, Antochshuk V, Cheung J, Mann L, Babadjanova Z, Rowand J, et al. . Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies. MAbs 2013; 5:787-94; PMID:23883920; http://dx.doi.org/10.4161/mabs.25269 - DOI - PMC - PubMed
-
- Razinkov V, Treuheit MJ, Becker GW. Methods of high throughput biophysical characterization in biopharmaceutical development. Curr Drug Discov Technol 2013; 10:59-70; PMID:22725690 - PubMed
-
- Eon-Duval A, Broly H, Gleixner R. Quality attributes of recombinant therapeutic proteins: an assessment of impact on safety and efficacy as part of a quality by design development approach. Biotechnol Prog 2012; 28:608-22; PMID:22473974; http://dx.doi.org/10.1002/btpr.1548 - DOI - PubMed
-
- He F, Woods CE, Becker GW, Narhi LO, Razinkov V. High-throughput assessment of thermal and colloidal stability parameters for monoclonal antibody formulations. J Pharm Sci 2011; 100:5126-41; PMID:21789772; http://dx.doi.org/10.1002/jps.22712 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
