A simplified score to quantify comorbidity in COPD
- PMID: 25514500
- PMCID: PMC4267736
- DOI: 10.1371/journal.pone.0114438
A simplified score to quantify comorbidity in COPD
Abstract
Importance: Comorbidities are common in COPD, but quantifying their burden is difficult. Currently there is a COPD-specific comorbidity index to predict mortality and another to predict general quality of life. We sought to develop and validate a COPD-specific comorbidity score that reflects comorbidity burden on patient-centered outcomes.
Materials and methods: Using the COPDGene study (GOLD II-IV COPD), we developed comorbidity scores to describe patient-centered outcomes employing three techniques: 1) simple count, 2) weighted score, and 3) weighted score based upon statistical selection procedure. We tested associations, area under the Curve (AUC) and calibration statistics to validate scores internally with outcomes of respiratory disease-specific quality of life (St. George's Respiratory Questionnaire, SGRQ), six minute walk distance (6MWD), modified Medical Research Council (mMRC) dyspnea score and exacerbation risk, ultimately choosing one score for external validation in SPIROMICS.
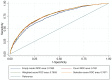
Results: Associations between comorbidities and all outcomes were comparable across the three scores. All scores added predictive ability to models including age, gender, race, current smoking status, pack-years smoked and FEV1 (p<0.001 for all comparisons). Area under the curve (AUC) was similar between all three scores across outcomes: SGRQ (range 0·7624-0·7676), MMRC (0·7590-0·7644), 6MWD (0·7531-0·7560) and exacerbation risk (0·6831-0·6919). Because of similar performance, the comorbidity count was used for external validation. In the SPIROMICS cohort, the comorbidity count performed well to predict SGRQ (AUC 0·7891), MMRC (AUC 0·7611), 6MWD (AUC 0·7086), and exacerbation risk (AUC 0·7341).
Conclusions: Quantifying comorbidity provides a more thorough understanding of the risk for patient-centered outcomes in COPD. A comorbidity count performs well to quantify comorbidity in a diverse population with COPD.
Conflict of interest statement
Figures
References
-
- Kochanek KD, Xu JQ, Murphy SL, Minino AM, Kung HC (2011) Deaths: Preliminary data for 2009. National vital statistics reports 59(4):1–51. - PubMed
-
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, et al. BODE Collaborative Group (2012) Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186(2):155–161. - PubMed
-
- Holguin F, Folch E, Redd SC, Mannino DM (2005) Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 128(4):2005–2011. - PubMed
-
- van Manen JG, Bindels PJ, Dekker EW, Ijzermans CJ, Bottema BJ, et al. (2001) Added value of co-morbidity in predicting health-related quality of life in COPD patients. Respir Med 95:496–504. - PubMed
Publication types
MeSH terms
Grants and funding
- 2R01HL089897/HL/NHLBI NIH HHS/United States
- HHSN268200900009C/WH/WHI NIH HHS/United States
- 2R01HL089856/HL/NHLBI NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- K23 HL103192/HL/NHLBI NIH HHS/United States
- T32HL007534/HL/NHLBI NIH HHS/United States
- R01 HL089856/HL/NHLBI NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- T32 HL007534/HL/NHLBI NIH HHS/United States
- R01 HL089897/HL/NHLBI NIH HHS/United States
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- S21 MD000101/MD/NIMHD NIH HHS/United States
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- HHSN2682009000019C/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases