A Winogradsky-based culture system shows an association between microbial fermentation and cystic fibrosis exacerbation
- PMID: 25514533
- PMCID: PMC4817692
- DOI: 10.1038/ismej.2014.234
A Winogradsky-based culture system shows an association between microbial fermentation and cystic fibrosis exacerbation
Erratum in
-
A Winogradsky-based culture system shows an association between microbial fermentation and cystic fibrosis exacerbation.ISME J. 2015 Mar 17;9(4):1052. doi: 10.1038/ismej.2014.266. ISME J. 2015. PMID: 25777574 Free PMC article. No abstract available.
Abstract
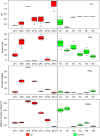
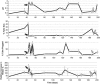
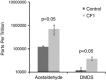
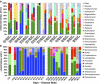
There is a poor understanding of how the physiology of polymicrobial communities in cystic fibrosis (CF) lungs contributes to pulmonary exacerbations and lung function decline. In this study, a microbial culture system based on the principles of the Winogradsky column (WinCF system) was developed to study the physiology of CF microbes. The system used glass capillary tubes filled with artificial sputum medium to mimic a clogged airway bronchiole. Chemical indicators were added to observe microbial physiology within the tubes. Characterization of sputum samples from seven patients showed variation in pH, respiration, biofilm formation and gas production, indicating that the physiology of CF microbial communities varied among patients. Incubation of homogenized tissues from an explant CF lung mirrored responses of a Pseudomonas aeruginosa pure culture, supporting evidence that end-stage lungs are dominated by this pathogen. Longitudinal sputum samples taken through two exacerbation events in a single patient showed that a two-unit drop in pH and a 30% increase in gas production occurred in the tubes prior to exacerbation, which was reversed with antibiotic treatment. Microbial community profiles obtained through amplification and sequencing of the 16S rRNA gene showed that fermentative anaerobes became more abundant during exacerbation and were then reduced during treatment where P. aeruginosa became the dominant bacterium. Results from the WinCF experiments support the model where two functionally different CF microbial communities exist, the persistent Climax Community and the acute Attack Community. Fermentative anaerobes are hypothesized to be the core members of the Attack Community and production of acidic and gaseous products from fermentation may drive developing exacerbations. Treatment targeting the Attack Community may better resolve exacerbations and resulting lung damage.
Figures


References
-
- Barker M, Hengst M, Schmid J, Buers H-J, Mittermaier B, Klemp D et al. (2006). Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur Respir J 27: 929–936. - PubMed
-
- Barth AL, Pitt TL. (1996). The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J Med Microbiol 45: 110–119. - PubMed
-
- Bjarnsholt T, Jensen PØ, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB et al. (2009). Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44: 547–558. - PubMed
-
- Burns JL, Rolain J-M. (2014). Culture-based diagnostic microbiology in cystic fibrosis: can we simplify the complexity? J Cyst Fibros 13: 1–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

