Single-dose pharmacokinetics of methylphenidate extended-release multiple layer beads administered as intact capsule or sprinkles versus methylphenidate immediate-release tablets (Ritalin(®)) in healthy adult volunteers
- PMID: 25514542
- PMCID: PMC4268571
- DOI: 10.1089/cap.2013.0135
Single-dose pharmacokinetics of methylphenidate extended-release multiple layer beads administered as intact capsule or sprinkles versus methylphenidate immediate-release tablets (Ritalin(®)) in healthy adult volunteers
Abstract
Objectives: The purpose of this study was to evaluate the relative bioavailability and safety of a multilayer extended-release bead methylphenidate (MPH) hydrochloride 80 mg (MPH-MLR) capsule or sprinkles (37% immediate-release [IR]) versus MPH hydrochloride IR(Ritalin(®)) tablets, and to develop a pharmacokinetic (PK) model simulating MPH concentration-time data for different MPH-MLR dosage strengths.
Methods: This was a single-center, randomized, open-label, three-period crossover study conducted in 26 fasted healthy adults (mean weight±standard deviation, 70.4±11.7 kg) assigned to single-dose oral MPH-MLR 80 mg capsule or sprinkles with applesauce, or Ritalin IR 25 mg (1×5 mg and 1×20 mg tablet) administered at 0, 4, and 8 hours.
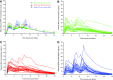
Results: MPH-MLR 80 mg capsule and sprinkles were bioequivalent; ratios for maximum concentration (Cmax), area under plasma drug concentration versus time curve (AUC)0-t, and AUC0-inf were 1.04 (95% confidence interval [CI], 96.3-112.4), 0.99 (95% CI, 95.3-102.8), and 0.99 (95% CI, 95.4-103.0), respectively. MPH-MLR capsule/sprinkles produced highly comparable, biphasic profiles of plasma MPH concentrations characterized by rapid initial peak, followed by moderate decline until 5 hours postdose, and gradual increase until 7 hours postdose, culminating in an attenuated second peak. Based on 90% CIs, total systemic exposure to MPH-MLR 80 mg capsule/sprinkles was similar to that for Ritalin IR 25 mg three times daily, but marked differences in Cmax values indicated that MPH-MLR regimens were not bioequivalent to Ritalin. MPH Cmax and total systemic exposure over the first 4 hours postdose with MPH-MLR capsule/sprinkles was markedly higher than that associated with the first dose of Ritalin. All study drugs were safe and well tolerated. The PK modeling in adults suggested that differences in MPH pharmacokinetics between MPH-MLR and Ritalin are the result of dosage form design attributes and the associated absorption profiles of MPH.
Conclusions: MPH-MLR 80 mg provides a long-acting biphasic pattern of plasma MPH concentrations with one less peak and trough than Ritalin IR.
Figures


References
-
- Banaschewski T, Coghill D, Santosh P, Zuddas A, Asherson P, Buitelaar J, Danckaerts M, Dopfner M, Faraone SV, Rothenberger A, Sergeant J, Steinhausen HC, Sonuga-Barke EJ, Taylor E: Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry 15:476–495, 2006 - PubMed
-
- Ermer JC, Adeyi BA, Pucci ML. Stimulants in the treatment of children and adults with attention-deficit hyperactivity disorder. CNS Drugs 24:1009–1025, 2010 - PubMed
-
- Faraone SV, Buitelaar J: Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry 19:353–364, 2010 - PubMed
-
- González MA, Pentikis HS, Anderl N, Benedict MF, DeCory HH, Dirksen SJ, Hatch SJ: Methylphenidate bioavailability from two extended-release formulations. Int J Clin Pharmacol Ther 40:175–184, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

