Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells
- PMID: 25514597
- PMCID: PMC4322987
- DOI: 10.18632/oncotarget.2589
Elevated endoplasmic reticulum stress reinforced immunosuppression in the tumor microenvironment via myeloid-derived suppressor cells
Abstract
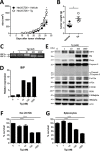
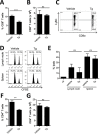
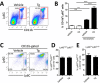
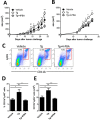
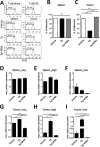
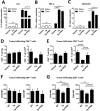
The role of endoplasmic reticulum (ER) stress in cancer has been studied in detail, and ER stress is known to increase tumor cell apoptosis, and thus, reduce tumor growth. However, in our study, persistent ER stress induced by multiple administrations of low-dose thapsigargin (Tg) accelerated tumor growth in mice. Tg-mediated ER stress increased the generation of Ly6G+CD11b+ myeloid cells, but did not alter anti-tumor effector T cells. 4-Phenylbutyric acid (4-PBA), a chemical chaperone widely used as an ER stress reducer, attenuated Tg-induced myeloid-derived suppressor cell (MDSC) expansion and tumor growth. Tg-mediated ER stress enhanced the immunosuppressive capacity of tumor-infiltrating MDSCs by increasing expression of ARG1, iNOS, and NOX2, although splenic MDSCs were not affected. Consistent with these results, 4-PBA restored the anti-tumor immune response by regulating inflammatory cytokines such as TNF-α and CXCL1/KC, and activated tumor-infiltrating CD8+ T cells that were inhibited by Tg-mediated ER stress. These results suggest that significant ER stress in a tumor-bearing host might induce tumor growth mediated by enhancement of MDSC-mediated suppression. Therefore, ER stress reducers such as 4-PBA could restore anti-tumor immunity by inhibiting suppressive MDSCs that are exacerbated by ER stress.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures
References
-
- Jackisch C, Hahm HA, Tombal B, McCloskey D, Butash K, Davidson NE, Denmeade SR. Delayed micromolar elevation in intracellular calcium precedes induction of apoptosis in thapsigargin-treated breast cancer cells. Clin Cancer Res. 2000;6:2844–2850. - PubMed
-
- Dubois C, Vanden Abeele F, Sehgal P, Olesen C, Junker S, Christensen SB, Prevarskaya N, Moller JV. Differential effects of thapsigargin analogues on apoptosis of prostate cancer cells: complex regulation by intracellular calcium. FEBS J. 2013;280:5430–5440. - PubMed
-
- Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. - PubMed
-
- Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–1828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

