TAM receptors support neural stem cell survival, proliferation and neuronal differentiation
- PMID: 25514676
- PMCID: PMC4267817
- DOI: 10.1371/journal.pone.0115140
TAM receptors support neural stem cell survival, proliferation and neuronal differentiation
Abstract
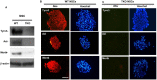
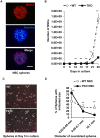
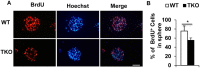
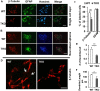
Tyro3, Axl and Mertk (TAM) receptor tyrosine kinases play multiple functional roles by either providing intrinsic trophic support for cell growth or regulating the expression of target genes that are important in the homeostatic regulation of immune responses. TAM receptors have been shown to regulate adult hippocampal neurogenesis by negatively regulation of glial cell activation in central nervous system (CNS). In the present study, we further demonstrated that all three TAM receptors were expressed by cultured primary neural stem cells (NSCs) and played a direct growth trophic role in NSCs proliferation, neuronal differentiation and survival. The cultured primary NSCs lacking TAM receptors exhibited slower growth, reduced proliferation and increased apoptosis as shown by decreased BrdU incorporation and increased TUNEL labeling, than those from the WT NSCs. In addition, the neuronal differentiation and maturation of the mutant NSCs were impeded, as characterized by less neuronal differentiation (β-tubulin III+) and neurite outgrowth than their WT counterparts. To elucidate the underlying mechanism that the TAM receptors play on the differentiating NSCs, we examined the expression profile of neurotrophins and their receptors by real-time qPCR on the total RNAs from hippocampus and primary NSCs; and found that the TKO NSC showed a significant reduction in the expression of both nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), but accompanied by compensational increases in the expression of the TrkA, TrkB, TrkC and p75 receptors. These results suggest that TAM receptors support NSCs survival, proliferation and differentiation by regulating expression of neurotrophins, especially the NGF.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

