PhosphoSitePlus, 2014: mutations, PTMs and recalibrations
- PMID: 25514926
- PMCID: PMC4383998
- DOI: 10.1093/nar/gku1267
PhosphoSitePlus, 2014: mutations, PTMs and recalibrations
Abstract
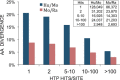
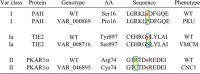
PhosphoSitePlus(®) (PSP, http://www.phosphosite.org/), a knowledgebase dedicated to mammalian post-translational modifications (PTMs), contains over 330,000 non-redundant PTMs, including phospho, acetyl, ubiquityl and methyl groups. Over 95% of the sites are from mass spectrometry (MS) experiments. In order to improve data reliability, early MS data have been reanalyzed, applying a common standard of analysis across over 1,000,000 spectra. Site assignments with P > 0.05 were filtered out. Two new downloads are available from PSP. The 'Regulatory sites' dataset includes curated information about modification sites that regulate downstream cellular processes, molecular functions and protein-protein interactions. The 'PTMVar' dataset, an intersect of missense mutations and PTMs from PSP, identifies over 25,000 PTMVars (PTMs Impacted by Variants) that can rewire signaling pathways. The PTMVar data include missense mutations from UniPROTKB, TCGA and other sources that cause over 2000 diseases or syndromes (MIM) and polymorphisms, or are associated with hundreds of cancers. PTMVars include 18 548 phosphorlyation sites, 3412 ubiquitylation sites, 2316 acetylation sites, 685 methylation sites and 245 succinylation sites.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Beausoleil S.A., Villen J., Gerber S.A., Rush J., Gygi S.P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006;24:1285–1292. - PubMed
-
- Miranda F.F., Teigen K., Thorolfsson M., Svebak R.M., Knappskog P.M., Flatmark T., Martinez A. Phosphorylation and mutations of Ser(16) in human phenylalanine hydroxylase. Kinetic and structural effects. J. Biol. Chem. 2002;277:40937–40943. - PubMed
-
- Gelmann E.P., Steadman D.J., Ma J., Ahronovitz N., Voeller H.J., Swope S., Abbaszadegan M., Brown K.M., Strand K., Hayes R.B., et al. Occurrence of NKX3.1 C154T polymorphism in men with and without prostate cancer and studies of its effect on protein function. Cancer Res. 2002;62:2654–2659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

