Intracranial pressure elevation after ischemic stroke in rats: cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy
- PMID: 25515213
- PMCID: PMC4420875
- DOI: 10.1038/jcbfm.2014.230
Intracranial pressure elevation after ischemic stroke in rats: cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy
Erratum in
-
Intracranial pressure elevation after ischemic stroke in rats: cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy.J Cereb Blood Flow Metab. 2015 Dec;35(12):2109. doi: 10.1038/jcbfm.2015.209. J Cereb Blood Flow Metab. 2015. PMID: 26621060 Free PMC article.
Abstract
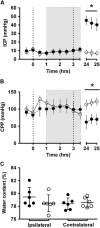
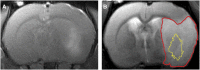
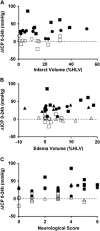
In both the human and animal literature, it has largely been assumed that edema is the primary cause of intracranial pressure (ICP) elevation after stroke and that more edema equates to higher ICP. We recently demonstrated a dramatic ICP elevation 24 hours after small ischemic strokes in rats, with minimal edema. This ICP elevation was completely prevented by short-duration moderate hypothermia soon after stroke. Here, our aims were to determine the importance of edema in ICP elevation after stroke and whether mild hypothermia could prevent the ICP rise. Experimental stroke was performed in rats. ICP was monitored and short-duration mild (35 °C) or moderate (32.5 °C) hypothermia, or normothermia (37 °C) was induced after stroke onset. Edema was measured in three studies, using wet-dry weight calculations, T2-weighted magnetic resonance imaging, or histology. ICP increased 24 hours after stroke onset in all normothermic animals. Short-duration mild or moderate hypothermia prevented this rise. No correlation was seen between ΔICP and edema or infarct volumes. Calculated rates of edema growth were orders of magnitude less than normal cerebrospinal fluid production rates. These data challenge current concepts and suggest that factors other than cerebral edema are the primary cause of the ICP elevation 24 hours after stroke onset.
Figures


References
-
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. - PubMed
-
- Ropper AH, Shafran B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch Neurol. 1984;41:26–29. - PubMed
-
- Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461–2466. - PubMed
-
- Morley NC, Berge E, Cruz-Flores S, Whittle IR. Surgical decompression for cerebral oedema in acute ischaemic stroke. Cochrane Database Syst Rev. 2002. - PubMed
-
- Murtha LA, McLeod DD, McCann SK, Pepperall D, Chung S, Levi CR, et al. Short-duration hypothermia after ischemic stroke prevents delayed intracranial pressure rise. Int J Stroke. 2014;9:553–559. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

