Sensory encoding in Neuregulin 1 mutants
- PMID: 25515311
- PMCID: PMC5040327
- DOI: 10.1007/s00429-014-0955-x
Sensory encoding in Neuregulin 1 mutants
Abstract
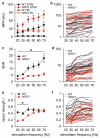
Schizophrenic patients show altered sensory perception as well as changes in electrical and magnetic brain responses to sustained, frequency-modulated sensory stimulation. Both the amplitude and temporal precision of the neural responses differ in patients as compared to control subjects, and these changes are most pronounced for stimulation at gamma frequencies (20-40 Hz). In addition, patients display enhanced spontaneous gamma oscillations, which has been interpreted as 'neural noise' that may interfere with normal stimulus processing. To investigate electrophysiological markers of aberrant sensory processing in a model of schizophrenia, we recorded neuronal activity in primary somatosensory cortex of mice heterozygous for the schizophrenia susceptibility gene Neuregulin 1. Sensory responses to sustained 20-70 Hz whisker stimulation were analyzed with respect to firing rates, spike precision (phase locking) and gamma oscillations, and compared to baseline conditions. The mutants displayed elevated spontaneous firing rates, a reduced gain in sensory-evoked spiking and gamma activity, and reduced spike precision of 20-40 Hz responses. These findings present the first in vivo evidence of the linkage between a genetic marker and altered stimulus encoding, thus suggesting a novel electrophysiological endophenotype of schizophrenia.
Keywords: Endophenotypes; Gamma oscillations; Neuregulin 1; Schizophrenia; Signal-to-noise ratio; Somatosensory cortex.
Figures


References
-
- Arnfred SM. Proprioceptive information processing in schizophrenia. Dan Med J. 2012:59. - PubMed
-
- Arnfred SM, Hemmingsen RP, Parnas J. Delayed early proprioceptive information processing in schizophrenia. Br J Psychiatry. 2006;189:558–559. - PubMed
-
- Arnfred SM, Morup M, Thalbitzer J, Jansson L, Parnas J. Attenuation of beta and gamma oscillations in schizophrenia spectrum patients following hand posture perturbation. Psychiatry Res. 2011;185:215–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

