Inhibition of the NLRP3 inflammasome provides neuroprotection in rats following amygdala kindling-induced status epilepticus
- PMID: 25516224
- PMCID: PMC4275944
- DOI: 10.1186/s12974-014-0212-5
Inhibition of the NLRP3 inflammasome provides neuroprotection in rats following amygdala kindling-induced status epilepticus
Abstract
Background: NLRP3 inflammasome is proposed to regulate inflammation in several neurological diseases, but its role in epilepsy remains largely unknown. This study aimed to investigate the role of the NLRP3 inflammasome in neuroinflammation, spontaneous recurrent seizures (SRS) and hippocampal neuronal loss in rat brain following amygdala kindling-induced status epilepticus (SE).
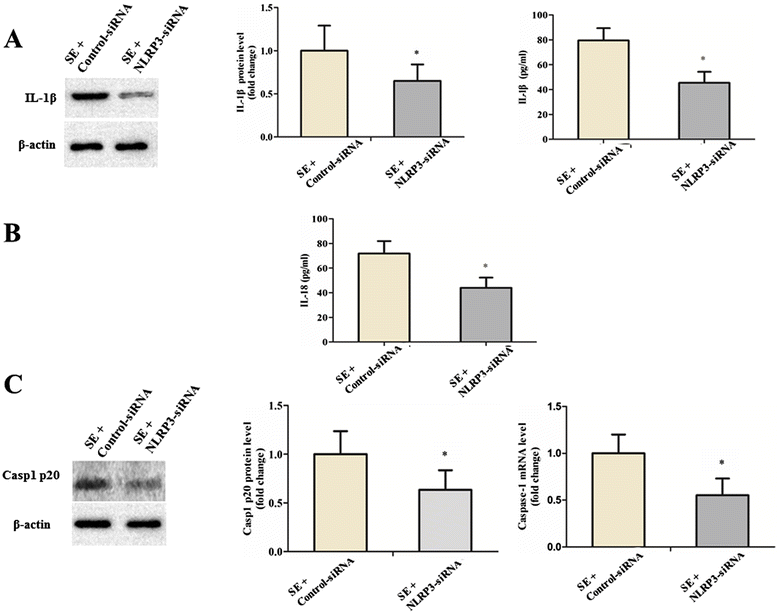
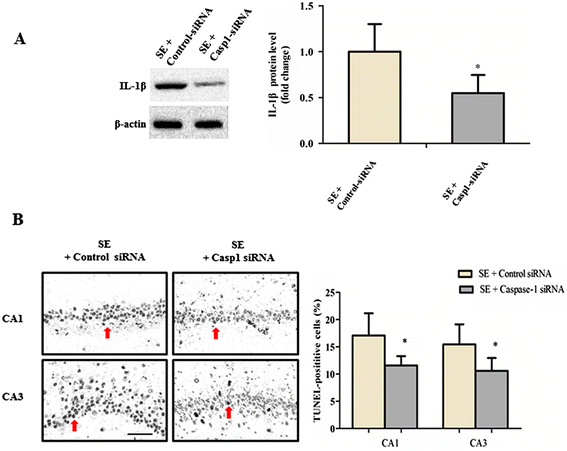
Methods: We detected the protein levels of IL-1β and NLRP3 inflammasome components by Western blot in the hippocampus of shams and SE rats at different time points following SE. To further examine whether the activation of the NLRP3 inflammasome contributes to SE-associated neuronal damage, we employed a nonviral strategy to knock down NLRP3 and caspase-1 expression in brain before undergoing SE. Proinflammatory cytokine levels and hippocampal neuronal loss were evaluated at 12 hours and at 6 weeks following SE respectively in these NLRP3 and caspase-1 deficient rats. Meanwhile, SRS occurrence was evaluated through a 4-week video recording started 2 weeks after SE in these NLRP3 and caspase-1 deficient rats.
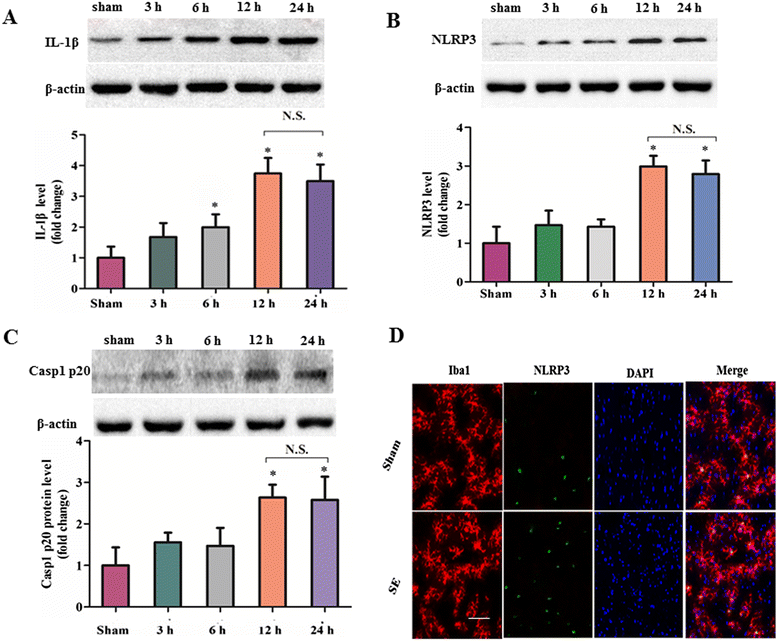
Results: IL-1β levels and NLRP3 inflammasome components levels dramatically increased at 3 hours after SE, and reached a maximum at 12 hours after SE compared with the control group. Knock down of NLRP3 or caspase-1 decreased the levels of IL-1β and IL-18 at 12 hours after SE, which was accompanied by a significant suppression in the development and severity of SRS during the chronic epileptic phase. Meanwhile, knock down of NLRP3 or caspase-1 led to a remarkable reduction of hippocampal neuronal loss in the CA1 and CA3 area of the hippocampus at 6 weeks after SE.
Conclusions: Our study provides the first evidence that the NLRP3 inflammasome was significantly up-regulated following SE. More importantly, we show that inhibition of the NLRP3 inflammasome provides neuroprotection in rats following SE. These findings suggest that NLRP3 may represent a potential target for the treatment of epileptogenesis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

