Exploring the folding pathway of green fluorescent protein through disulfide engineering
- PMID: 25516354
- PMCID: PMC4353360
- DOI: 10.1002/pro.2621
Exploring the folding pathway of green fluorescent protein through disulfide engineering
Abstract
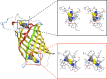
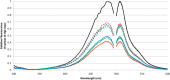
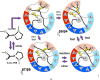
We have introduced two disulfide crosslinks into the loop regions on opposite ends of the beta barrel in superfolder green fluorescent protein (GFP) in order to better understand the nature of its folding pathway. When the disulfide on the side opposite the N/C-termini is formed, folding is 2× faster, unfolding is 2000× slower, and the protein is stabilized by 16 kJ/mol. But when the disulfide bond on the side of the termini is formed we see little change in the kinetics and stability. The stabilization upon combining the two crosslinks is approximately additive. When the kinetic effects are broken down into multiple phases, we observe Hammond behavior in the upward shift of the kinetic m-value of unfolding. We use these results in conjunction with structural analysis to assign folding intermediates to two parallel folding pathways. The data are consistent with a view that the two fastest transition states of folding are "barrel closing" steps. The slower of the two phases passes through an intermediate with the barrel opening occurring between strands 7 and 8, while the faster phase opens between 9 and 4. We conclude that disulfide crosslink-induced perturbations in kinetics are useful for mapping the protein folding pathway.
Keywords: folding pathway; kinetics; leave-one-out GFP; protein design.
© 2014 The Protein Society.
Figures


References
-
- Jones LN, Simon M, Watts NR, Booy FP, Steven AC, Parry DAD. Intermediate filament structure: hard α-keratin. Biophys Chem. 1997;68:83–93. - PubMed
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. - PubMed
-
- Ruoppolo M, Vinci F, Klink TA, Raines RT, Marino G. Contribution of individual disulfide bonds to the oxidative folding of Ribonuclease A. Biochemistry. 2000;39:12033–12042. - PubMed
-
- Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta, Proteins Proteomics. 2004;1699:35–44. - PubMed
-
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

