Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs
- PMID: 25516419
- PMCID: PMC4345798
- DOI: 10.1136/thoraxjnl-2014-206225
Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs
Abstract
Rationale: The molecular mechanisms underlying the muscle atrophy of intensive care unit-acquired weakness (ICUAW) are poorly understood. We hypothesised that increased circulating and muscle growth and differentiation factor-15 (GDF-15) causes atrophy in ICUAW by changing expression of key microRNAs.
Objectives: To investigate GDF-15 and microRNA expression in patients with ICUAW and to elucidate possible mechanisms by which they cause muscle atrophy in vivo and in vitro.
Methods: In an observational study, 20 patients with ICUAW and seven elective surgical patients (controls) underwent rectus femoris muscle biopsy and blood sampling. mRNA and microRNA expression of target genes were examined in muscle specimens and GDF-15 protein concentration quantified in plasma. The effects of GDF-15 on C2C12 myotubes in vitro were examined.
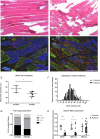
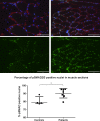
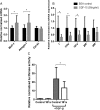
Measurements and main results: Compared with controls, GDF-15 protein was elevated in plasma (median 7239 vs 2454 pg/mL, p=0.001) and GDF-15 mRNA in the muscle (median twofold increase p=0.006) of patients with ICUAW. The expression of microRNAs involved in muscle homeostasis was significantly lower in the muscle of patients with ICUAW. GDF-15 treatment of C2C12 myotubes significantly elevated expression of muscle atrophy-related genes and down-regulated the expression of muscle microRNAs. miR-181a suppressed transforming growth factor-β (TGF-β) responses in C2C12 cells, suggesting increased sensitivity to TGF-β in ICUAW muscle. Consistent with this suggestion, nuclear phospho-small mothers against decapentaplegic (SMAD) 2/3 was increased in ICUAW muscle.
Conclusions: GDF-15 may increase sensitivity to TGF-β signalling by suppressing the expression of muscle microRNAs, thereby promoting muscle atrophy in ICUAW. This study identifies both GDF-15 and associated microRNA as potential therapeutic targets.
Keywords: Not Applicable; Respiratory Muscles.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures




References
-
- De Jonghe B, Sharshar T, Lefaucheur J, et al. . Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859–67. - PubMed
-
- Batt J, dos Santos CC, Cameron JI, et al. . Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med 2013;187:238–46. - PubMed
-
- Puthucheary ZA, Rawal J, McPhail M, et al. . Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600. - PubMed
-
- Bloch S, Polkey MI, Griffiths M, et al. . Molecular mechanisms of intensive care unit-acquired weakness. Eur Respir J 2012;39:1000–11. - PubMed
-
- Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev 2013;24:373–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
