Identification of β-hematin inhibitors in a high-throughput screening effort reveals scaffolds with in vitro antimalarial activity
- PMID: 25516843
- PMCID: PMC4266794
- DOI: 10.1016/j.ijpddr.2014.08.002
Identification of β-hematin inhibitors in a high-throughput screening effort reveals scaffolds with in vitro antimalarial activity
Abstract
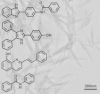
The emergence of drug resistant strains of Plasmodium spp. creates a critical need for the development of novel antimalarials. Formation of hemozoin, a crystalline heme detoxification process vital to parasite survival serves as an important drug target. The quinoline antimalarials including chloroquine and amodiaquine owe their antimalarial activity to inhibition of hemozoin formation. Though in vivo formation of hemozoin occurs within the presence of neutral lipids, the lipophilic detergent NP-40 was previously shown to serve as a surrogate in the β-hematin (synthetic hemozoin) formation process. Consequently, an NP-40 mediated β-hematin formation assay was developed for use in high-throughput screening. Here, the assay was utilized to screen 144,330 compounds for the identification of inhibitors of crystallization, resulting in 530 hits. To establish the effectiveness of these target-based β-hematin inhibitors against Plasmodium falciparum, each hit was further tested in cultures of parasitized red blood cells. This effort revealed that 171 of the β-hematin inhibitors are also active against the parasite. Dose-response data identified 73 of these β-hematin inhibitors have IC50 values ⩽5 μM, including 25 compounds with nanomolar activity against P. falciparum. A scaffold-based analysis of this data identified 14 primary scaffolds that represent 46% of the 530 total hits. Representative compounds from each of the classes were further assessed for hemozoin inhibitory activity in P. falciparum infected human erythrocytes. Each of the hit compounds tested were found to be positive inhibitors, while a negative control did not perturb this biological pathway in culture.
Keywords: Hemozoin; High-throughput screen; Malaria; Plasmodium falciparum; Scaffolds; β-Haematin.
Figures



References
-
- Cheeseman I.H., Miller B.A., Nair S., Nkhoma S., Tan A., Tan J.C., Al Saai S., Phyo A.P., Moo C.L., Lwin K.M., McGready R., Ashley E., Imwong M., Stepniewska K., Yi P., Dondorp A.M., Mayxay M., Newton P.N., White N.J., Nosten F., Ferdig M.T., Anderson T.J. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336:79–82. - PMC - PubMed
-
- De D., Krogstad F.M., Cogswell F.B., Krogstad D.J. Aminoquinolines that circumvent resistance in Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 1996;55:579–583. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

