Mga is essential for the survival of pluripotent cells during peri-implantation development
- PMID: 25516968
- PMCID: PMC4299147
- DOI: 10.1242/dev.111104
Mga is essential for the survival of pluripotent cells during peri-implantation development
Abstract
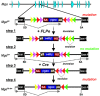
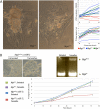
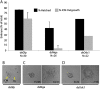
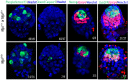
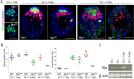
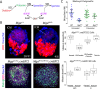
The maintenance and control of pluripotency is of great interest in stem cell biology. The dual specificity T-box/basic-helix-loop-helix-zipper transcription factor Mga is expressed in the pluripotent cells of the inner cell mass (ICM) and epiblast of the peri-implantation mouse embryo, but its function has not been investigated previously. Here, we use a loss-of-function allele and RNA knockdown to demonstrate that Mga depletion leads to the death of proliferating pluripotent ICM cells in vivo and in vitro, and the death of embryonic stem cells (ESCs) in vitro. Additionally, quiescent pluripotent cells lacking Mga are lost during embryonic diapause. Expression of Odc1, the rate-limiting enzyme in the conversion of ornithine into putrescine in the synthesis of polyamines, is reduced in Mga mutant cells, and the survival of mutant ICM cells as well as ESCs is rescued in culture by the addition of exogenous putrescine. These results suggest a mechanism whereby Mga influences pluripotent cell survival through regulation of the polyamine pool in pluripotent cells of the embryo, whether they are in a proliferative or quiescent state.
Keywords: Basic-helix-loop-helix-zipper; ESCs; Mga; Mouse; ODC; Pluripotency; T-box; Transcription factor.
© 2015. Published by The Company of Biologists Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

