Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis
- PMID: 25517096
- PMCID: PMC4342003
- DOI: 10.1038/nature14064
Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis
Abstract
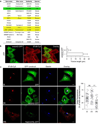
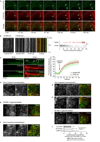
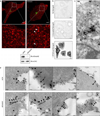
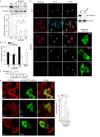
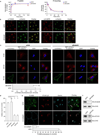
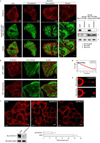
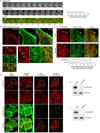
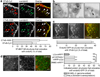
During endocytosis, energy is invested to narrow the necks of cargo-containing plasma membrane invaginations to radii at which the opposing segments spontaneously coalesce, thereby leading to the detachment by scission of endocytic uptake carriers. In the clathrin pathway, dynamin uses mechanical energy from GTP hydrolysis to this effect, assisted by the BIN/amphiphysin/Rvs (BAR) domain-containing protein endophilin. Clathrin-independent endocytic events are often less reliant on dynamin, and whether in these cases BAR domain proteins such as endophilin contribute to scission has remained unexplored. Here we show, in human and other mammalian cell lines, that endophilin-A2 (endoA2) specifically and functionally associates with very early uptake structures that are induced by the bacterial Shiga and cholera toxins, which are both clathrin-independent endocytic cargoes. In controlled in vitro systems, endoA2 reshapes membranes before scission. Furthermore, we demonstrate that endoA2, dynamin and actin contribute in parallel to the scission of Shiga-toxin-induced tubules. Our results establish a novel function of endoA2 in clathrin-independent endocytosis. They document that distinct scission factors operate in an additive manner, and predict that specificity within a given uptake process arises from defined combinations of universal modules. Our findings highlight a previously unnoticed link between membrane scaffolding by endoA2 and pulling-force-driven dynamic scission.
Figures



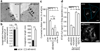

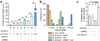
Comment in
-
Cell biology: On the endocytosis rollercoaster.Nature. 2015 Jan 22;517(7535):446-7. doi: 10.1038/nature14081. Epub 2014 Dec 17. Nature. 2015. PMID: 25517097 No abstract available.
-
Endocytosis. A new gateway into cells.Nat Rev Mol Cell Biol. 2015 Feb;16(2):68. doi: 10.1038/nrm3939. Nat Rev Mol Cell Biol. 2015. PMID: 25604192
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

