The kinetic stability of cytochrome C oxidase: effect of bound phospholipid and dimerization
- PMID: 25517159
- PMCID: PMC4269771
- DOI: 10.1016/j.bpj.2014.10.055
The kinetic stability of cytochrome C oxidase: effect of bound phospholipid and dimerization
Abstract
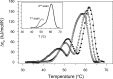
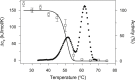
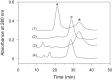
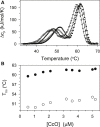
Thermally induced transitions of the 13-subunit integral membrane protein bovine cytochrome c oxidase (CcO) have been studied by differential scanning calorimetry (DSC) and circular dichroism (CD). Thermal denaturation of dodecyl maltoside solubilized CcO proceeds in two consecutive, irreversible, kinetically driven steps with the apparent transition temperatures at ∼ 51°C and ∼ 61°C (5μM CcO at scan rate of 1.5 K/min). The thermal denaturation data were analyzed according to the Lyubarev and Kurganov model of two consecutive irreversible steps. However, because of the limitation of the model to describe the complex mechanism of the thermal denaturation of CcO, the obtained results were utilized only for comparison purposes of kinetic stabilities of CcO under specific protein concentration (5μM) and scan rate (1.5 K/min). This enabled us to show that both the amphiphilic environment and the self-association state of CcO affect its kinetic stability. Kinetic stabilities of both steps are significantly decreased when all of the phospholipids are removed from CcO by phospholipase A2 (the half-life decreases at 37°C). Conversely, dimerization of CcO induced by sodium cholate significantly increases its kinetic stability of only the first step (the half-life increases at 37°C). Protein concentration-dependent nonspecific oligomerization also indicate mild stabilization of CcO. Both, reversed-phase high-performance liquid chromatography (HPLC) and SDS-PAGE subunit analysis reveal that the first step of thermal denaturation involves dissociation of subunits III, VIa, VIb, and VIIa, whereas the second step is less well defined and most likely involves global unfold and aggregation of the remaining subunits. Electron transport activity of CcO decreases in a sigmoidal manner during the first transition and this dependence is very well described by kinetic parameters for the first step of the thermal transition. Therefore, dissociation of subunit III and/or VIIa is responsible for temperature-induced inactivation of CcO because VIa and VIb can be removed from CcO without affecting the enzyme activity. These results demonstrate an important role of tightly bound phospholipids and oligomeric state (particularly the dimeric form) of CcO for kinetic stability of the protein.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Kadenbach B., Jarausch J., Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal. Biochem. 1983;129:517–521. - PubMed
-
- Tsukihara T., Aoyama H., Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–1144. - PubMed
-
- Sedlák E., Robinson N.C. Phospholipase A(2) digestion of cardiolipin bound to bovine cytochrome c oxidase alters both activity and quaternary structure. Biochemistry. 1999;38:14966–14972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

