Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics
- PMID: 25517309
- PMCID: PMC4623410
- DOI: 10.4161/mabs.29445
Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics
Abstract
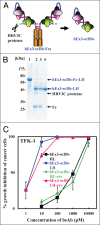
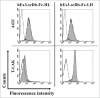
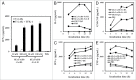
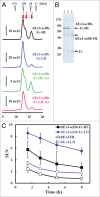
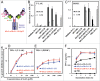
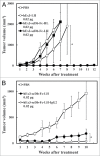
One approach to creating more beneficial therapeutic antibodies is to develop bispecific antibodies (bsAbs), particularly IgG-like formats with tetravalency, which may provide several advantages such as multivalent binding to each target antigen. Although the effects of configuration and antibody-fragment type on the function of IgG-like bsAbs have been studied, there have been only a few detailed studies of the influence of the variable fragment domain order. Here, we prepared four types of hEx3-scDb-Fc, IgG-like bsAbs, built from a single-chain hEx3-Db (humanized bispecific diabody [bsDb] that targets epidermal growth factor receptor and CD3), to investigate the influence of domain order and fusion manner on the function of a bsDb with an Fc fusion format. Higher cytotoxicities were observed with hEx3-scDb-Fcs with a variable light domain (VL)-variable heavy domain (VH) order (hEx3-scDb-Fc-LHs) compared with a VH-VL order, indicating that differences in the Fc fusion manner do not affect bsDb activity. In addition, flow cytometry suggested that the higher cytotoxicities of hEx3-scDb-Fc-LH may be attributable to structural superiority in cross-linking. Interestingly, enhanced degradation resistance and prolonged in vivo half-life were also observed with hEx3-scDb-Fc-LH. hEx3-scDb-Fc-LH and its IgG2 variant exhibited intense in vivo antitumor effects, suggesting that Fc-mediated effector functions are dispensable for effective anti-tumor activities, which may cause fewer side effects. Our results show that merely rearranging the domain order of IgG-like bsAbs can enhance not only their antitumor activity, but also their degradation resistance and in vivo half-life, and that hEx3-scDb-Fc-LHs are potent candidates for next-generation therapeutic antibodies.
Keywords: ADCC, antibody-dependent cell-mediated cytotoxicity; AUC, area-under-the-curve; CD3; EGFR, epidermal growth factor receptor; FITC-CD3ϵγ, fluorescein isothiocyanate-labeled CD3ϵγ; DVD-IgTM, dual variable domain immunoglobulin; FITC-sEGFR, FITC-labeled sEGFR; Fv, variable fragment; ICR, imprinting control region; IgG-like bispecific antibody; MTS, 3-(4, 5-dimethylthiazole-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt; PBMCs, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SPR, surface plasmon resonance; SUV, standardized uptake value; T-LAK cells, lymphokine-activated killer cells with the T-cell phenotype; VH, variable heavy domain; VL, variable light domain; antibody engineering; bispecific diabody; bsAb, bispecific antibody; bsDb, bispecific diabody; cancer immunotherapy; effective domain order; epidermal growth factor receptor; sEGFR, soluble EGFR; scDb, single-chain diabody; scFv, single-chain Fv; taFv, tandem scFv.
Figures


References
-
- Byrne H, Conroy PJ, Whisstock JC, O’Kennedy RJ. A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol 2013; 31:621-32; PMID:24094861; http://dx.doi.org/10.1016/j.tibtech.2013.08.007 - DOI - PMC - PubMed
-
- Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs 2009; 1:539-47; PMID:20073127; http://dx.doi.org/10.4161/mabs.1.6.10015 - DOI - PMC - PubMed
-
- Holliger P, Winter G. Diabodies: small bispecific antibody fragments. Cancer Immunol Immunother 1997; 45:128-30; PMID:9435855; http://dx.doi.org/10.1007/s002620050414 - DOI - PMC - PubMed
-
- Stork R, Campigna E, Robert B, Müller D, Kontermann RE. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J Biol Chem 2009; 284:25612-9; PMID:19628871; http://dx.doi.org/10.1074/jbc.M109.027078 - DOI - PMC - PubMed
-
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et al. . Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321:974-7; PMID:18703743; http://dx.doi.org/10.1126/science.1158545 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
