A monoclonal antibody against hinge-cleaved IgG restores effector function to proteolytically-inactivated IgGs in vitro and in vivo
- PMID: 25517311
- PMCID: PMC4623506
- DOI: 10.4161/mabs.29825
A monoclonal antibody against hinge-cleaved IgG restores effector function to proteolytically-inactivated IgGs in vitro and in vivo
Abstract
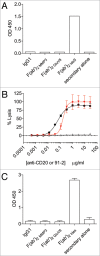
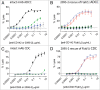
We report a chimeric monoclonal antibody (mAb) directed to a neo-epitope that is exposed in the IgG lower hinge following proteolytic cleavage. The mAb, designated 2095-2, displays specificity for IdeS-generated F(ab')₂ fragments, but not for full-length IgG or for closely-related F(ab')₂ fragments generated with other proteases. A critical component of the specificity is provided by the C-terminal amino acid of the epitope corresponding to gly-236 in the IgG1 (also IgG4) hinge. By its ability to bind to IdeS-cleaved anti-CD20 mAb, mAb 2095-2 fully restored antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) against WIL2-S cells to the otherwise inactive anti-CD20 IgG1 F(ab')₂ fragment. Similarly, 2095-2 reinstated ADCC against MDA-MB-231 cells to an anti-CD142 IgG1 F(ab')₂ fragment. mAb 2095-2 was also capable of eliciting both CDC and ADCC to IgG4 F(ab')₂ fragments, an IgG subclass that has weaker ADCC and CDC when intact relative to intact IgG1. The in vitro cell-based efficacy of 2095-2 was extended to the in vivo setting using platelets as a cell clearance surrogate. In a canine model, the co-administration of 2095-2 together with IdeS-generated, platelet-targeting anti-CD41/61 F(ab')₂ fragment not only restored platelet clearance, but did so at a rate and extent of clearance that exceeded that of intact anti-CD41/61 IgG at comparable concentrations. To further explore this unexpected amplification effect, we conducted a rat study in which 2095-2 was administered at a series of doses in combination with a fixed dose of anti-CD41/61 F(ab')₂ fragments. Again, the combination, at ratios as low as 1:10 (w/w) 2095-2 to F(ab')₂, proved more effective than the anti-CD41/61 IgG1 alone. These findings suggest a novel mechanism for enhancing antibody-mediated cell-killing effector functions with potential applications in pathologic settings such as tumors and acute infections where protease activity is abundant.
Keywords: ADCC, antibody-dependent cell-mediated cytotoxicity; CDC, complement-dependent cytotoxicity; FACS, fluorescence-activated cell sorter; GluV8, glutamyl endopeptidase V8; IdeS, Immunoglobulin G-degrading enzyme of Streptococcus pyogenes; IgG fragments; MMP, matrix metalloproteinase; PBMC, peripheral blood mononuclear cell; antibody-dependent cell-mediated cytotoxicity; chimeric antibody; complement-dependent cytotoxicity; hinge region; mAb, monoclonal antibody.
Figures



References
-
- Brezski RJ, Knight DM, Jordan RE. The origins, specificity, and potential biological relevance of human anti-IgG hinge autoantibodies. ScientificWorldJournal 2011; 11:1153-67; PMID:21623461; http://dx.doi.org/10.1100/tsw.2011.107 - DOI - PMC - PubMed
-
- Brezski RJ, Luongo JL, Petrone D, Ryan MH, Zhong D, Tam SH, Schmidt AP, Kruszynski M, Whitaker BP, Knight DM, et al. . Human anti-IgG1 hinge autoantibodies reconstitute the effector functions of proteolytically inactivated IgGs. J Immunol 2008; 181:3183-92; PMID:18713989; http://dx.doi.org/10.4049/jimmunol.181.5.3183 - DOI - PubMed
-
- Brezski RJ, Jordan RE. Cleavage of IgGs by proteases associated with invasive diseases: an evasion tactic against host immunity? MAbs 2010; 2:212-20; PMID:20400859; http://dx.doi.org/10.4161/mabs.2.3.11780 - DOI - PMC - PubMed
-
- von Pawel-Rammingen U. Streptococcal IdeS and its impact on immune response and inflammation. J Innate Immun 2012; 4:132-40; PMID:22248585; http://dx.doi.org/10.1159/000332940 - DOI - PMC - PubMed
-
- Brezski RJ, Oberholtzer A, Strake B, Jordan RE. The in vitro resistance of IgG2 to proteolytic attack concurs with a comparative paucity of autoantibodies against peptide analogs of the IgG2 hinge. MAbs 2011; 3:558-67; PMID:22123056; http://dx.doi.org/10.4161/mabs.3.6.18119 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
