Noninvasive Quantitative Imaging of Collagen Microstructure in Three-Dimensional Hydrogels Using High-Frequency Ultrasound
- PMID: 25517512
- PMCID: PMC4499776
- DOI: 10.1089/ten.TEC.2014.0527
Noninvasive Quantitative Imaging of Collagen Microstructure in Three-Dimensional Hydrogels Using High-Frequency Ultrasound
Abstract
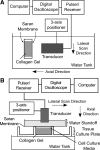
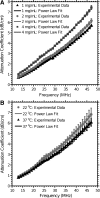
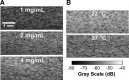
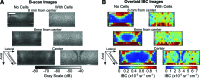
Collagen I is widely used as a natural component of biomaterials for both tissue engineering and regenerative medicine applications. The physical and biological properties of fibrillar collagens are strongly tied to variations in collagen fiber microstructure. The goal of this study was to develop the use of high-frequency quantitative ultrasound to assess collagen microstructure within three-dimensional (3D) hydrogels noninvasively and nondestructively. The integrated backscatter coefficient (IBC) was employed as a quantitative ultrasound parameter to detect, image, and quantify spatial variations in collagen fiber density and diameter. Collagen fiber microstructure was varied by fabricating hydrogels with different collagen concentrations or polymerization temperatures. IBC values were computed from measurements of the backscattered radio-frequency ultrasound signals collected using a single-element transducer (38-MHz center frequency, 13-47 MHz bandwidth). The IBC increased linearly with increasing collagen concentration and decreasing polymerization temperature. Parametric 3D images of the IBC were generated to visualize and quantify regional variations in collagen microstructure throughout the volume of hydrogels fabricated in standard tissue culture plates. IBC parametric images of corresponding cell-embedded collagen gels showed cell accumulation within regions having elevated collagen IBC values. The capability of this ultrasound technique to noninvasively detect and quantify spatial differences in collagen microstructure offers a valuable tool to monitor the structural properties of collagen scaffolds during fabrication, to detect functional differences in collagen microstructure, and to guide fundamental research on the interactions of cells and collagen matrices.
Figures






Similar articles
-
Controlling collagen fiber microstructure in three-dimensional hydrogels using ultrasound.J Acoust Soc Am. 2013 Aug;134(2):1491-502. doi: 10.1121/1.4812868. J Acoust Soc Am. 2013. PMID: 23927189 Free PMC article.
-
Estimating cell concentration in three-dimensional engineered tissues using high frequency quantitative ultrasound.Ann Biomed Eng. 2014 Jun;42(6):1292-304. doi: 10.1007/s10439-014-0994-8. Epub 2014 Mar 14. Ann Biomed Eng. 2014. PMID: 24627179 Free PMC article.
-
Noninvasive, quantitative, spatiotemporal characterization of mineralization in three-dimensional collagen hydrogels using high-resolution spectral ultrasound imaging.Tissue Eng Part C Methods. 2012 Dec;18(12):935-46. doi: 10.1089/ten.TEC.2012.0180. Epub 2012 Jul 16. Tissue Eng Part C Methods. 2012. PMID: 22624791 Free PMC article.
-
Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery.Chem Soc Rev. 2015 Aug 7;44(15):5031-9. doi: 10.1039/c5cs00278h. Epub 2015 May 20. Chem Soc Rev. 2015. PMID: 25992492 Review.
-
Hydrogels for Engineering of Perfusable Vascular Networks.Int J Mol Sci. 2015 Jul 14;16(7):15997-6016. doi: 10.3390/ijms160715997. Int J Mol Sci. 2015. PMID: 26184185 Free PMC article. Review.
Cited by
-
In vivo acoustic patterning of endothelial cells for tissue vascularization.Sci Rep. 2023 Sep 26;13(1):16082. doi: 10.1038/s41598-023-43299-0. Sci Rep. 2023. PMID: 37752255 Free PMC article.
-
Medical imaging of tissue engineering and regenerative medicine constructs.Biomater Sci. 2021 Jan 21;9(2):301-314. doi: 10.1039/d0bm00705f. Epub 2020 Aug 10. Biomater Sci. 2021. PMID: 32776044 Free PMC article. Review.
-
Ultrasound patterning technologies for studying vascular morphogenesis in 3D.J Cell Sci. 2017 Jan 1;130(1):232-242. doi: 10.1242/jcs.188151. Epub 2016 Oct 27. J Cell Sci. 2017. PMID: 27789577 Free PMC article.
-
Within or Without You? A Perspective Comparing In Situ and Ex Situ Tissue Engineering Strategies for Articular Cartilage Repair.Adv Healthc Mater. 2022 Dec;11(24):e2201305. doi: 10.1002/adhm.202201305. Epub 2022 Nov 22. Adv Healthc Mater. 2022. PMID: 36541723 Free PMC article.
-
A novel quantitative and reference-free ultrasound analysis to discriminate different concentrations of bone mineral content.Sci Rep. 2021 Jan 11;11(1):301. doi: 10.1038/s41598-020-79365-0. Sci Rep. 2021. PMID: 33432022 Free PMC article.
References
-
- Achilli M., and Mantovani D. Tailoring mechanical properties of collagen-based scaffolds for vascular tissue engineering: the effects of ph, temperature and ionic strength on gelation. Polymers Basel 2, 664, 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

