Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice
- PMID: 25517879
- PMCID: PMC4615674
- DOI: 10.4161/19490976.2014.990784
Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice
Abstract
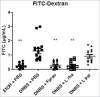
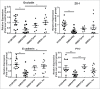
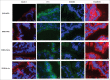
Impaired gut barrier function has been reported in a wide range of diseases and syndromes and in some functional gastrointestinal disorders. In addition, there is increasing evidence that suggests the gut microbiota tightly regulates gut barrier function and recent studies demonstrate that probiotic bacteria can enhance barrier integrity. Here, we aimed to investigate the effects of Lactobacillus rhamnosus CNCM I-3690 on intestinal barrier function. In vitro results using a Caco-2 monolayer cells stimulated with TNF-α confirmed the anti-inflammatory nature of the strain CNCM I-3690 and pointed out a putative role for the protection of the epithelial function. Next, we tested the protective effects of L. rhamnosus CNCM I-3690 in a mouse model of increased colonic permeability. Most importantly, we compared its performance to that of the well-known beneficial human commensal bacterium Faecalibacterium prauznitzii A2-165. Increased colonic permeability was normalized by both strains to a similar degree. Modulation of apical tight junction proteins expression was then analyzed to decipher the mechanism underlying this effect. We showed that CNCM I-3690 partially restored the function of the intestinal barrier and increased the levels of tight junction proteins Occludin and E-cadherin. The results indicate L. rhamnosus CNCM I-3690 is as effective as the commensal anti-inflammatory bacterium F. prausnitzii to treat functional barrier abnormalities.
Keywords: AJs, adherence junctions; CNCM, Collection Nationale de Cultures de Microorganismes; DNBS, DiNitroBenzene Sulfonic; EOS, extremely oxygen sensitive; GVHD, graft-versus-host disease; IBD, inflammatory bowel diseases; IBS, irritable bowel syndrome; LAB, lac; apical junction proteins; lactobacilli; probiotics.
Figures




References
-
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013; 70:631-59; PMID:22782113; http://dx.doi.org/ 10.1007/s00018-012-1070-x - DOI - PMC - PubMed
-
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008; 88:249-86; PMID:18195088; http://dx.doi.org/ 10.1152/physrev.00018.2006 - DOI - PubMed
-
- Kiela PR, Ghishan FK. Ion transport in the intestine. Curr Opin Gastroenterol 2009; 25:87-91; PMID:19528875; http://dx.doi.org/ 10.1097/MOG.0b013e3283260900 - DOI - PMC - PubMed
-
- Bradley M D, Sanjay K Nigam. Molecular structure and assembly of the tight junction. Am J Physiol Renal Physiol 1998; 274:1-9. - PubMed
-
- Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 2013; 69:42-51; PMID:23089410; http://dx.doi.org/ 10.1016/j.phrs.2012.10.007 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
