Transcriptional alterations of ET-1 axis and DNA damage in lung tissue of a rat obesity model
- PMID: 25517973
- PMCID: PMC4337466
- DOI: 10.1089/dna.2014.2705
Transcriptional alterations of ET-1 axis and DNA damage in lung tissue of a rat obesity model
Abstract
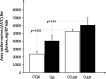
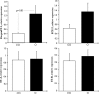
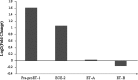
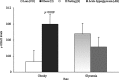
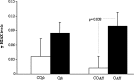
Obesity has been implicated in the development of many cancers. This can lead to genome damage, especially in the form of double-strand break, the presence of which is now easily detected through nuclear phosphorylation of histone H2AX (γ-H2AX) focus assay. Recently, the endothelin (ET) axis has also been shown to have a role in the growth and progression of several tumors, including lung cancer. The aim of this study was to evaluate the ET-1 system transcriptional alterations and γ-H2AX in lung tissue of Zucker rats subdivided into obese (O, n=22) and controls (CO, n=18) rats: under either fasting conditions (CO(fc)-O(fc)) or acute hyperglycemia (CO(AH)-O(AH)). Significantly higher prepro-ET-1 (p=0.05) and ET-converting enzyme (ECE)-2 mRNA expression was observed in O with respect to CO. A significant positive association was observed between prepro-ET-1 and ET-A in the whole rat population (p=0.009) or in the obese group alone (p=0.007). The levels of γ-H2AX in O and in O(AH) rats were significantly higher (p=0.019) than in the corresponding CO and CO(AH) rats (p=0.038). The study shows an inappropriate secretion of ET-1 in O animals with a parallel DNA damage in their lungs, providing novel mechanisms by which ET receptor antagonist may exert organ protection.
Figures



References
-
- Ahme S.I., Thompson J., Coulson J.M., and Woll P. (2000). Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol 22,422–431 - PubMed
-
- Bagnato A., Salani D., Di Castro V., Wu-Wong J.R., Tecce R., Nicotra M.R., Venuti A., and Ntali P.G. (1999). Expression of endothelin 1 and endothelin-A receptor ovarian carcinoma evidence for an autocrine role in tumor growth. Cancer Res 59,720–727 - PubMed
-
- Bartkova J., Horejsi Z., Koed K., Kramer A., Tort F., Ziegerm K., Guldberg P., Sehested M., Nesland J.M., Lukasm C., Orntoft T., Lukas J., and Bartek J. (2005). DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434,864–870 - PubMed
-
- Battistini B., Chailer P., D'Orleans-Juste P., Briere N., and Sirois P. (1993). Growth regulatory properties of endothelins. Peptides 14,385–399 - PubMed
-
- Bhalla A., Haque S., Taylor I., Winslet M., and Loizidou M. (2009). Endothelin receptor antagonism and cancer. Eur J Clin Invest 39,74–77 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

