Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production
- PMID: 25519186
- PMCID: PMC4326729
- DOI: 10.1095/biolreprod.114.126326
Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production
Abstract
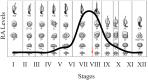
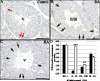
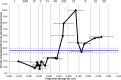
The asynchronous cyclic nature of spermatogenesis is essential for continual sperm production and is one of the hallmarks of mammalian male fertility. While various mRNA and protein localization studies have indirectly implicated changing retinoid levels along testis tubules, no quantitative evidence for these changes across the cycle of the seminiferous epithelium currently exists. This study utilized a unique mouse model of induced synchronous spermatogenesis, localization of the retinoid-signaling marker STRA8, and sensitive quantification of retinoic acid concentrations to determine whether there are fluctuations in retinoid levels at each of the individual stages of germ cell differentiation and maturation to sperm. These data show that processive pulses of retinoic acid are generated during spermatogonial differentiation and are the likely trigger for cyclic spermatogenesis and allow us, for the first time, to understand how the cycle of the seminiferous epithelium is generated and maintained. In addition, this study represents the first direct quantification of a retinoid gradient controlling cellular differentiation in a postnatal tissue.
Keywords: retinoic acid; spermatogenesis; spermatogonia; testis.
© 2015 by the Society for the Study of Reproduction, Inc.
Figures




References
-
- Begemann G, Meyer A. Hindbrain patterning revisited: timing and effects of retinoic acid signalling. Bioessays. 2001;23:981–986. - PubMed
-
- Briggs LJ, Stein D, Goltz J, Corrigan VC, Efthymiadis A, Hubner S, Jans DA. The cAMP-dependent protein kinase site (Ser312) enhances dorsal nuclear import through facilitating nuclear localization sequence/importin interaction. J Biol Chem. 1998;273:22745–22752. - PubMed
-
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. - PubMed
-
- Towers M, Wolpert L, Tickle C. Gradients of signalling in the developing limb. Curr Opin Cell Biol. 2012;24:181–187. - PubMed
-
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

