Comparative performance assessment of point-of-care testing devices for measuring glucose and ketones at the patient bedside
- PMID: 25519295
- PMCID: PMC4604596
- DOI: 10.1177/1932296814563351
Comparative performance assessment of point-of-care testing devices for measuring glucose and ketones at the patient bedside
Abstract
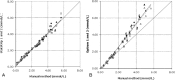
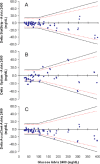
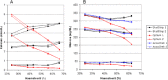
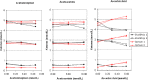
Point-of-care (POC) testing devices for monitoring glucose and ketones can play a key role in the management of dysglycemia in hospitalized diabetes patients. The accuracy of glucose devices can be influenced by biochemical changes that commonly occur in critically ill hospital patients and by the medication prescribed. Little is known about the influence of these factors on ketone POC measurements. The aim of this study was to assess the analytical performance of POC hospital whole-blood glucose and ketone meters and the extent of glucose interference factors on the design and accuracy of ketone results. StatStrip glucose/ketone, Optium FreeStyle glucose/ketone, and Accu-Chek Performa glucose were also assessed and results compared to a central laboratory reference method. The analytical evaluation was performed according to Clinical and Laboratory Standards Institute (CLSI) protocols for precision, linearity, method comparison, and interference. The interferences assessed included acetoacetate, acetaminophen, ascorbic acid, galactose, maltose, uric acid, and sodium. The accuracies of both Optium ketone and glucose measurements were significantly influenced by varying levels of hematocrit and ascorbic acid. StatStrip ketone and glucose measurements were unaffected by the interferences tested with exception of ascorbic acid, which reduced the higher level ketone value. The accuracy of Accu-Chek glucose measurements was affected by hematocrit, by ascorbic acid, and significantly by galactose. The method correlation assessment indicated differences between the meters in compliance to ISO 15197 and CLSI 12-A3 performance criteria. Combined POC glucose/ketone methods are now available. The use of these devices in a hospital setting requires careful consideration with regard to the selection of instruments not sensitive to hematocrit variation and presence of interfering substances.
Keywords: glucose; ketones; performance evaluation; point-of-care testing.
© 2014 Diabetes Technology Society.
Conflict of interest statement
Figures

References
-
- Fahy BG, Sheehy AM, Coursin DB. Glucose control in the intensive care unit. Crit Care Med. 2009;37:1769-1776. - PubMed
-
- Alter D, Deines G. Tight glycemic control and point-of-care testing. Clin Lab Med. 2009;29:511-522. - PubMed
-
- Prisco F, Picardi A, Iafusco D, et al. Blood ketone bodies in patients with recent-onset type 1 diabetes (a multicenter study). Pediatr Diabetes. 2006;7:223-228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

