Interventions for fatigue in peripheral neuropathy
- PMID: 25519471
- PMCID: PMC10793713
- DOI: 10.1002/14651858.CD008146.pub2
Interventions for fatigue in peripheral neuropathy
Abstract
Background: Persistent feelings of fatigue (or subjective fatigue), which may be experienced in the absence of physiological factors, affect many people with peripheral neuropathy. A variety of interventions for subjective fatigue are available, but little is known about their efficacy or the likelihood of any adverse effects for people with peripheral neuropathy.
Objectives: To assess the effects of drugs and physical, psychological or behavioural interventions for fatigue in adults or children with peripheral neuropathy.
Search methods: On 5 November 2013, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL, MEDLINE, EMBASE, CINAHL Plus, LILACS and AMED. We also searched reference lists of all studies identified for inclusion and relevant reviews, and contacted the authors of included studies and known experts in the field to identify additional published or unpublished data. We also searched trials registries for ongoing studies.
Selection criteria: We considered for inclusion randomised controlled trials (RCTs) and quasi-RCTs comparing any form of intervention for fatigue management in adults with peripheral neuropathy with placebo, no intervention or an alternative form of intervention for fatigue. Interventions considered included drugs, pacing and grading of physical activity, general or specific exercise, compensatory strategies such as orthotics, relaxation, counselling, cognitive and educational strategies.
Data collection and analysis: Two review authors independently assessed risk of bias and extracted study data. We contacted study authors for additional information. We collected information on adverse events from the included trials.
Main results: The review includes three trials, which were all at low risk of bias, involving 530 people with peripheral neuropathy. The effects of amantadine from one randomised, double-blind, placebo-controlled, cross-over trial comparing amantadine with placebo for the treatment of fatigue in 80 people with Guillain-Barré syndrome (GBS) were uncertain for the proportion of people achieving a favourable outcome six weeks post-intervention (odds ratio (OR) 0.56 (95% confidence interval (CI) 0.22 to 1.35, N = 74, P = 0.16). We assessed the quality of this evidence as low. Two parallel-group randomised double-blind, placebo-controlled trials comparing the effects of two doses of ascorbic acid with placebo for reducing fatigue in adults with Charcot-Marie-Tooth disease type 1A (CMT1A) showed that the effects of ascorbic acid at either dose are probably small (standardised mean difference (SMD) -0.12 (95% CI -0.32 to 0.08, n = 404, P = 0.25)) for change in fatigue after 12 to 24 months (moderate quality evidence). Neither ascorbic acid study measured fatigue at four to 12 weeks, which was our primary outcome measure. No serious adverse events were reported with amantadine. Serious adverse events were reported in the trials of ascorbic acid. However,risk of serious adverse events was similar with ascorbic acid and placebo.
Authors' conclusions: One small imprecise study in people with GBS showed uncertain effects of amantadine on fatigue. In two studies in people with CMT1A there is moderate-quality evidence that ascorbic acid has little meaningful benefit on fatigue. Information about adverse effects was limited, although both treatments appear to be well tolerated and safe in these conditions.There was no evidence available from RCTs to evaluate the effect of other drugs or other interventions for fatigue in either GBS, CMT1A or other causes of peripheral neuropathy. The cost effectiveness of different interventions should also be considered in future randomised clinical trials.
Conflict of interest statement
One author (CW) is currently conducting a single‐blind randomised controlled trial of tailored home exercise for people with inflammatory neuropathy (Home exercise for Inflammatory Neuropathy Trial ‐ HINT). The trial is funded by the Guillain‐Barré syndrome support group UK (GBSSG). CW is on the Medical Advisory Board of the GBSSG UK. She has no known conflicts of interest.
Pieter A van Doorn (PAvD) and his institution have received consultancy fees from Talecris and CSL Behring for membership of the Scientific board of the ICE trial in CIDP and a scientific board on IVIg in chronic polyneuropathy. PAvD's department has received research grants from Baxter, Sanquin and Talecris: 1. A grant to conduct a RCT comparing IVIg versus IVIg and steroids in Guillain‐Barré syndrome; 2. A grant to conduct a RCT investigating the effect of a second course of IVIg (SID‐trial) in Guillain‐Barré syndrome patients with a poor prognosis; 3. A grant to conduct a prospective international study on the effect of a second course of IVIg in Guillain‐Barré syndrome patients with a poor prognosis. MPG and PAvD have co‐ordinated or conducted one of the trials that was eligible for inclusion in the review (Garssen 2006).
Marcel P Garssen has no known financial conflicts of interest.
Rachel Stockley has no known conflicts of interest.
Figures


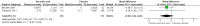
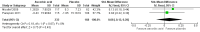







Update of
References
References to studies included in this review
Garssen 2006 {published data only}
-
- Garssen MPJ, Schmitz PIM, Merkies ISJ, Jacobs BC, Meché FGA, Doorn PA. Amantadine for treatment of fatigue in Guillain‐Barré syndrome: a randomised, double blind, placebo controlled, crossover trial. Journal of Neurology, Neurosurgery and Psychiatry 2006;77(1):61‐5. [PUBMED: 16361594] - PMC - PubMed
Micallef 2009 {published data only}
-
- Micallef J, Attarian S, Dubourg O, Gonnaud P‐M, Hogrel J‐Y, Stojkovic T, et al. Effect of ascorbic acid in patients with Charcot‐Marie‐Tooth disease type 1A: a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet Neurology 2009;8(12):1103‐10. [PUBMED: 19818690] - PubMed
References to studies excluded from this review
Burns 2009 {published data only}
-
- Burns J, Ouvrier RA, Yiu EM, Joseph PD, Kornberg AJ, Fahey MC, et al. Ascorbic acid for Charcot‐Marie‐Tooth disease type 1A in children: a randomised, double‐blind, placebo‐controlled safety and efficacy trial. Lancet Neurology 2009;8(6):537‐44. - PubMed
Carter 2006 {published data only}
-
- Carter GT, Han JJ, Mayadev A, Weiss MD. Modafinil reduces fatigue in Charcot‐Marie‐Tooth disease type 1A: a case series. American Journal of Hospice and Palliative Care 2006;23(5):412‐6. - PubMed
Enderlin 2008 {published data only}
-
- Enderlin CA. Peripheral neuropathy, sleep and fatigue related to cancer and chemotherapy: preliminary findings. Southern Online Journal of Nursing Research 2008;8(2):2p.
NCT00484510 {published and unpublished data}
-
- NCT00484510. A randomized, placebo‐controlled, double masked 120 subject "futility design" clinical trial of ascorbic acid treatment of Charcot Marie Tooth disease type 1A. clinicaltrials.gov/ct2/show/NCT00484510 (accessed 21 January 2013).
References to ongoing studies
Attarian 2013 {published data only}
-
- Attarian S, Dubourg O, Funalot B, Gonnaud P‐M, Lacour A, Magy L, et al. A phase II randomized, placebo‐controlled multicenter clinical trial of three doses of PXT3003 in 80 adult patients with CMT1A treated for 1 year. Journal of the Peripheral Nervous System 2013;18(s2):S7‐S8.
NCT00541164 {published data only}
-
- NCT00541164. Effects of coenzyme Q10 on Charcot‐Marie‐Tooth disease. //clinicaltrials.gov/ct2/show/NCT00541164 (accessed 12 June 2014).
NCT02121678 {published data only}
-
- NCT02121678. Effect of resistance and aerobic exercise in CIDP or MMN. www.clinicaltrials.gov/ct2/show/NCT02121678 (accessed 12 June 2014).
Ramdharry 2011 {published data only}
-
- Ramdharry GM, Pollard AJ, Anderson CA, Laurá M, Murphy SM, Hutton EJ, et al. Strengthening hip flexors to improve walking distance in people with Charcot‐Marie‐Tooth disease. Journal of the Peripheral Nervous System. 2011; Vol. 16 (Suppl 3):S115‐6.
Additional references
Bernardy 2013
Bigland‐Ritchie 1978
-
- Bigland‐Ritchie B, Jones DA, Hosking GP, Edwards RH. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clinical Science and Molecular Medicine 1978;54(6):609‐14. - PubMed
Bleijenberg 2003
Busch 2007
Cappelen‐Smith 2000
-
- Cappelen‐Smith C, Kuwabara S, Lin CS, Mogyoros I, Burke D. Activity‐dependent hyperpolarization and conduction block in chronic inflammatory demyelinating polyneuropathy. Annals of Neurology 2000;48(6):826‐32. - PubMed
Chalder 1993
-
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. Journal of Psychosomatic Research 1993;37(2):147‐53. - PubMed
Cohen 1989
-
- Cohen RA, Fisher M. Amantadine treatment of fatigue associated with multiple sclerosis. Archives of Neurology 1989;46(6):676‐80. - PubMed
Cramp 2012
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Edmonds 2004
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues.. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Fisk 1994
-
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clinical Infectious Diseases 1994;18(Suppl 1):S79‐83. - PubMed
Fisk 2002
-
- Fisk JD, Doble SE. Construction and validation of a fatigue impact scale for daily administration (D‐FIS). Quality of Life Research 2002;11(3):263‐72. - PubMed
Gandevia 2001
-
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiological Reviews 2001;81(4):1725‐89. - PubMed
Garssen 2004
-
- Garssen MP, Bussmann JB, Schmitz PI, Zandbergen A, Welter TG, Merkies IS, et al. Physical training and fatigue, fitness, and quality of life in Guillain‐Barré syndrome and CIDP. Neurology 2004;63(12):2393‐5. - PubMed
Garssen 2007
-
- Garssen MP, Schillings ML, Doorn PA, Engelen BG, Zwarts MJ. Contribution of central and peripheral factors to residual fatigue in Guillain‐Barré syndrome. Muscle & Nerve 2007;36(1):93‐9. - PubMed
GRADEpro 2008 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Graham 2007
-
- Graham RC, Hughes RAC, White CM. A prospective study of physiotherapist prescribed community based exercise in inflammatory peripheral neuropathy. Journal of Neurology 2007;254(2):228‐35. - PubMed
Hauser 2013
Henningsen 2003
-
- Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta‐analytic review. Psychosomatic Medicine 2003;65(4):528‐33. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kaji 2000
-
- Kaji R, Bostock H, Kohara N, Murase N, Kimura J, Shibasaki H. Activity‐dependent conduction block in multifocal motor neuropathy. Brain 2000;123(Pt 8):1602‐11. - PubMed
Karlsen 1999
-
- Karlsen K, Larsen JP, Tandberg E, Jorgensen K. Fatigue in patients with Parkinson's disease. Movement Disorders 1999;14(2):237‐41. - PubMed
Kent‐Braun 1993
-
- Kent‐Braun JA, Sharma KR, Weiner MW, Massie B, Miller RG. Central basis of muscle fatigue in chronic fatigue syndrome. Neurology 1993;43(1):125‐31. - PubMed
Kleyweg 1991
-
- Kleyweg RP, Meché FGA, Schmitz PIM. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain‐Barre syndrome. Muscle & Nerve 1991;14(11):1103‐9. - PubMed
Krupp 1989
-
- Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 1989;46(10):1121‐3. - PubMed
Krupp 2003
-
- Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs 2003;17(4):225‐34. - PubMed
Lee 1991
-
- Lee KA, Hicks G, Nino‐Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research 1991;36(3):291‐8. - PubMed
Lennon 1993
-
- Lennon SM, Koblar S, Hughes RAC, Goeller J, Riser AC. Reasons for persistent disability in Guillain‐Barré syndrome. Clinical Rehabilitation 1993;7(1):1‐8.
Martinez‐Figuerora 1977
Martyn 1998
-
- Martyn C, Hughes RAC. Peripheral neuropathies. The Epidemiology of Neurological Disorders. 1st Edition. London: BMJ, 1998.
Merkies 1999
-
- Merkies IS, Schmitz PI, Samijn JP, Meché FG, Doorn PA. Fatigue in immune‐mediated polyneuropathies. European Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 1999;53(8):1648‐54. - PubMed
Minton 2010
Moss‐Morris 2005
-
- Moss‐Morris R, Sharon C, Tobin R, Baldi JC. A randomized controlled graded exercise trial for chronic fatigue syndrome: outcomes and mechanisms of change. Journal of Health Psychology 2005;10(2):245‐9. - PubMed
Pfeiffer 2001
Piper 1998
-
- Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncology Nursing Forum 1998;25(4):677‐84. - PubMed
Price 2008
Pucci 2009
Rosenberg 1988
-
- Rosenberg GA, Appenzeller O. Amantadine, fatigue, and multiple sclerosis. Archives of Neurology 1988;45(10):1104‐6. - PubMed
Sanders 1996
-
- Sanders DB, Stalberg EV, Nandedkar SD. Analysis of the electromyographic interference pattern. Journal of Clinical Neurophysiology 1996;13(5):385‐400. - PubMed
Schillings 2003
-
- Schillings ML, Hoefsloot W, Stegeman DF, Zwarts MJ. Relative contributions of central and peripheral factors to fatigue during a maximal sustained effort. European Journal of Applied Physiology 2003;90(5‐6):562‐8. - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions.. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tamura 2007
-
- Tamura N, Kuwabara S, Misawa S, Kanai K, Nakata M, Sawai S, et al. Time course of axonal regeneration in acute motor axonal neuropathy. Muscle & Nerve 2007;35(6):793‐5. - PubMed
Tejani 2012
van Nes 2009
-
- Nes SI, Vanhoutte EK, Faber CG, Garssen M, Doorn PA, Merkies IS, PeriNomS Study Group. Improving fatigue assessment in immune‐mediated neuropathies: the modified Rasch‐built Fatigue Severity Scale. Journal of the Peripheral Nervous System 2009;14(4):268‐78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

