Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales
- PMID: 25519478
- PMCID: PMC4302440
- DOI: 10.1186/s12955-014-0187-z
Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales
Abstract
Background: Single-item assessments have been the most often-used measures in National Cancer Institute (NCI) cancer control clinical trials, but normative data are not available. Our objective was to examine the normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scale for overall quality of life (QOL).
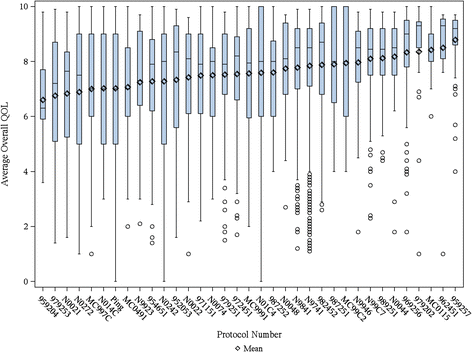
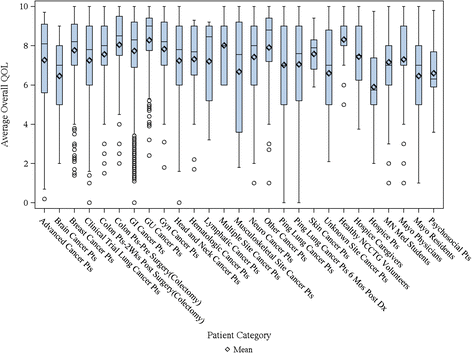
Methods: We analyzed baseline data from 36 clinical trials and 6 observational studies with various populations, including healthy volunteers, cancer trial patients (patients with advanced incurable cancer or patients receiving treatment with curative intent) and hospice patients as well as their caregivers. The overall QOL LASA was rated 0 (as bad as it can be) to 10 (as good as it can be). We calculated the summary statistics and the proportion of patients reporting a clinically meaningful deficit (CMD) of a score equal to 5 or less on the 0-10 scale.
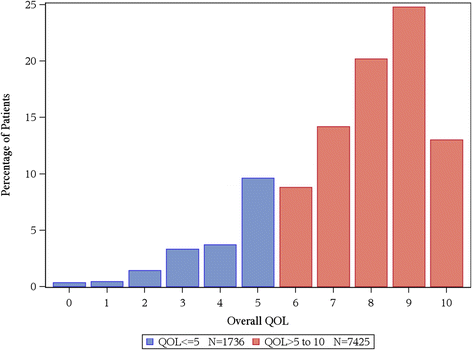
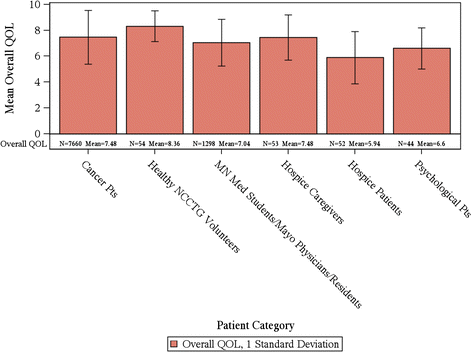
Results: In total, for the collective sample of 9,295 individuals, the average overall QOL reported was 7.39 (SD = 2.11) with a markedly skewed distribution with roughly 17% reporting a score of 5 or below indicating a clinically significant deficit in overall QOL. Hospice patients report a much worse average score of 5.7 upon entry to hospice; hospice caregivers average 7.4. Cancer patients vary within these two extremes with most patients averaging in the 7's on the 0-10 scale (range, 0 to 10 p-value < 0.0001). Men and women's QOL distributions were virtually identical (with average of 7.6 vs. 7.5, p-value = 0.046). Overall QOL was weakly related to performance status with a Spearman correlation coefficient of -0.29 (p-value < 0.0001). Overall QOL was related to tumor response (p-value = 0.0094), i.e. patients with a full or partial response reported a CMD in 11.4% of cases compared to 14.4% among those with stable disease and 18.5% among those with disease progression. Data missingness was high for performance status and tumor response associations.
Conclusions: This study provides the normative data for cancer patients and healthy volunteers for overall QOL using the LASA. These can serve as benchmarks for future studies and inform clinical practice decision-making.
Figures
Similar articles
-
Patient-reported outcomes version of the common terminology criteria for adverse events and quality-of-life linear analogue self-assessment in breast cancer patients receiving radiation therapy: single-institution prospective registry.J Patient Rep Outcomes. 2022 Jan 10;6(1):3. doi: 10.1186/s41687-021-00408-9. J Patient Rep Outcomes. 2022. PMID: 35006393 Free PMC article.
-
Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients.J Pain Symptom Manage. 2007 Dec;34(6):628-38. doi: 10.1016/j.jpainsymman.2007.01.016. Epub 2007 Aug 20. J Pain Symptom Manage. 2007. PMID: 17703910 Free PMC article.
-
Prospective study of quality of life in adults with newly diagnosed high-grade gliomas.J Neurooncol. 2006 Feb;76(3):283-91. doi: 10.1007/s11060-005-7020-9. J Neurooncol. 2006. PMID: 16163448
-
Exercise interventions for adults with cancer receiving radiation therapy alone.Cochrane Database Syst Rev. 2023 Mar 13;3(3):CD013448. doi: 10.1002/14651858.CD013448.pub2. Cochrane Database Syst Rev. 2023. PMID: 36912791 Free PMC article. Review.
-
Active mind-body movement therapies as an adjunct to or in comparison with pulmonary rehabilitation for people with chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2018 Oct 10;10(10):CD012290. doi: 10.1002/14651858.CD012290.pub2. Cochrane Database Syst Rev. 2018. PMID: 30306545 Free PMC article.
Cited by
-
Outcomes of a Dignity Therapy/Life Plan Intervention for Patients With Advanced Cancer Undergoing Chemotherapy.J Hosp Palliat Nurs. 2018 Aug;20(4):400-406. doi: 10.1097/NJH.0000000000000461. J Hosp Palliat Nurs. 2018. PMID: 30063634 Free PMC article.
-
Correlates of Successful Aging in Racial and Ethnic Minority Women Age 80 Years and Older: Findings from the Women's Health Initiative.J Gerontol A Biol Sci Med Sci. 2016 Mar;71 Suppl 1(Suppl 1):S87-99. doi: 10.1093/gerona/glv099. J Gerontol A Biol Sci Med Sci. 2016. PMID: 26858329 Free PMC article.
-
What patient-reported outcome measures may be suitable for research involving older adults with frailty? A scoping review.Eur Geriatr Med. 2024 Jun;15(3):629-644. doi: 10.1007/s41999-024-00964-5. Epub 2024 Mar 26. Eur Geriatr Med. 2024. PMID: 38532081 Free PMC article.
-
Healthy immigrant community study protocol: A randomized controlled trial of a social network intervention for cardiovascular risk reduction among Hispanic and Somali adults.Contemp Clin Trials. 2024 Mar;138:107465. doi: 10.1016/j.cct.2024.107465. Epub 2024 Feb 2. Contemp Clin Trials. 2024. PMID: 38309526 Free PMC article.
-
Prognostic impact of patient-reported symptoms in multiple myeloma.Blood Adv. 2025 Feb 25;9(4):884-892. doi: 10.1182/bloodadvances.2024014232. Blood Adv. 2025. PMID: 39637309 Free PMC article.
References
-
- Brundage M, Blazeby J, Revicki D, Bass B, de Vet H, Duffy H, Efficace F, King M, Lam CL, Moher D, Scott J, Sloan J, Snyder C, Yount S, Calvert M. Patient-reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Qual Life Res. 2013;22(6):1161–75. doi: 10.1007/s11136-012-0252-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

