An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A
- PMID: 25519680
- PMCID: PMC4311411
- DOI: 10.1186/s13023-014-0199-0
An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A
Erratum in
-
Erratum to: An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A.Orphanet J Rare Dis. 2016 Jul 7;11(1):92. doi: 10.1186/s13023-016-0463-6. Orphanet J Rare Dis. 2016. PMID: 27387831 Free PMC article. No abstract available.
Abstract
Background: Charcot-Marie-Tooth type 1A disease (CMT1A) is a rare orphan inherited neuropathy caused by an autosomal dominant duplication of a gene encoding for the structural myelin protein PMP22, which induces abnormal Schwann cell differentiation and dysmyelination, eventually leading to axonal suffering then loss and muscle wasting. We favour the idea that diseases can be more efficiently treated when targeting multiple disease-relevant pathways. In CMT1A patients, we therefore tested the potential of PXT3003, a low-dose combination of three already approved compounds (baclofen, naltrexone and sorbitol). Our study conceptually builds on preclinical experiments highlighting a pleiotropic mechanism of action that includes downregulation of PMP22. The primary objective was to assess safety and tolerability of PXT3003. The secondary objective aimed at an exploratory analysis of efficacy of PXT3003 in CMT1A, to be used for designing next clinical development stages (Phase 2b/3).
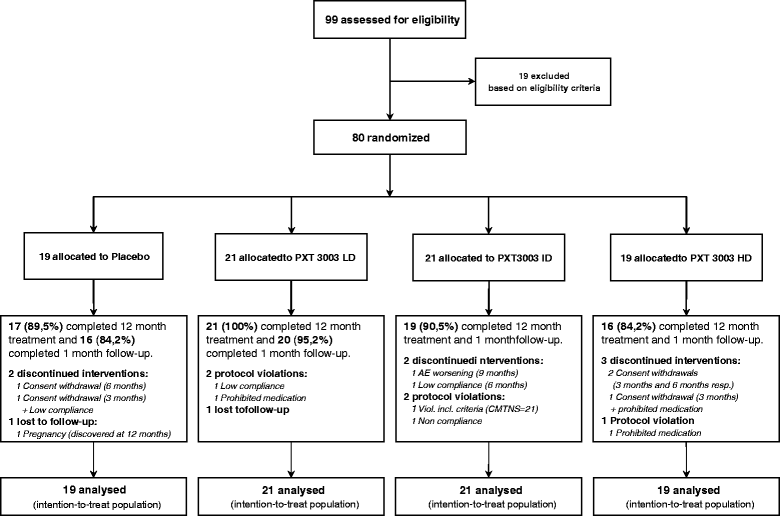
Methods: 80 adult patients with mild-to-moderate CMT1A received in double-blind for 1 year Placebo or one of the three increasing doses of PXT3003 tested, in four equal groups. Safety and tolerability were assessed with the incidence of related adverse events. Efficacy was assessed using the Charcot-Marie-Tooth Neuropathy Score (CMTNS) and the Overall Neuropathy Limitations Scale (ONLS) as main endpoints, as well as various clinical and electrophysiological outcomes.
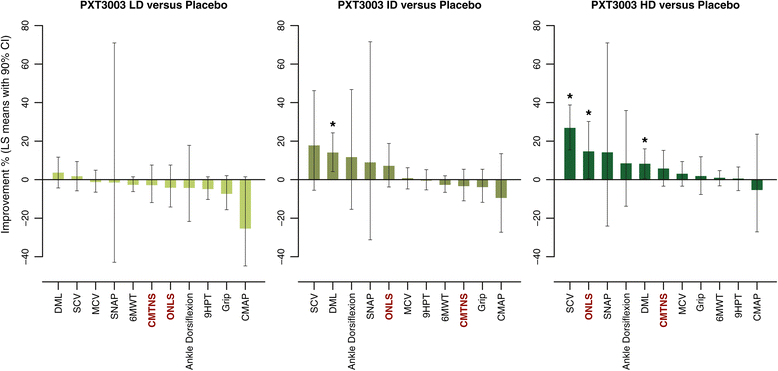
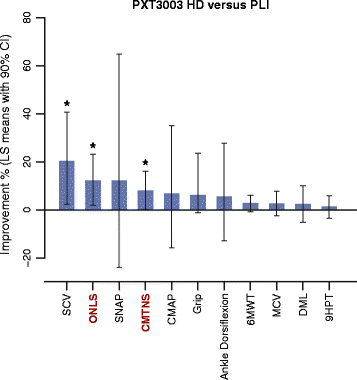
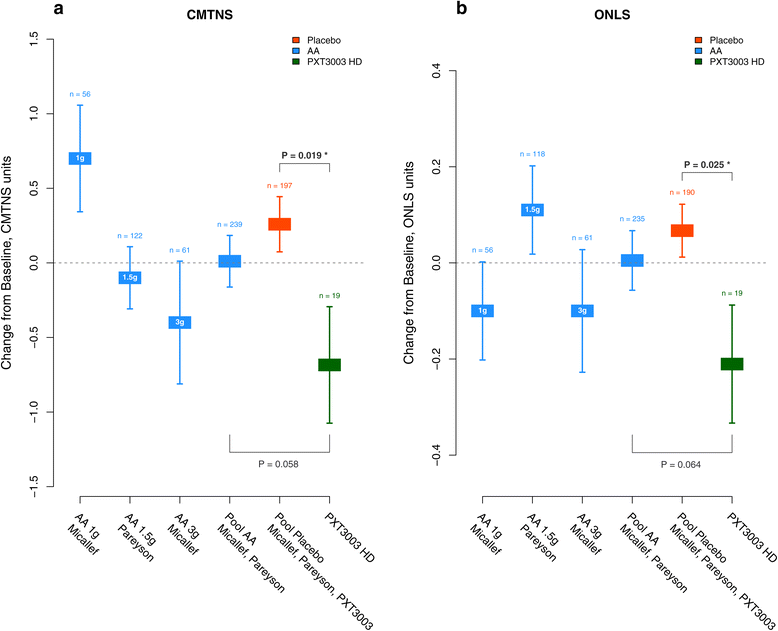
Results: This trial confirmed the safety and tolerability of PXT3003. The highest dose (HD) showed consistent evidence of improvement beyond stabilization. CMTNS and ONLS, with a significant improvement of respectively of 8% (0.4% - 16.2%) and 12.1% (2% - 23.2%) in the HD group versus the pool of all other groups, appear to be the most sensitive clinical endpoints to treatment despite their quasi-stability over one year under Placebo. Patients who did not deteriorate over one year were significantly more frequent in the HD group.
Conclusions: These results confirm that PXT3003 deserves further investigation in adults and could greatly benefit CMT1A-diagnosed children, usually less affected than adults.
Trial registration: EudraCT Number: 2010-023097-40. ClinicalTrials.gov Identifier: NCT01401257. The Committee for Orphan Medicinal Products issued in February 2014 a positive opinion on the application for orphan designation for PXT3003 (EMA/OD/193/13).
Figures

References
-
- Kochański A. Molecular genetics studies in polish Charcot-Marie-Tooth families. Folia Neuropathol. 2005;42:65–73. - PubMed
-
- Bird TD. ᅟ. 2014. Charcot-Marie-Tooth Neuropathy Type 1.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

