Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation
- PMID: 25519950
- PMCID: PMC6457828
- DOI: 10.1002/14651858.CD011335.pub2
Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation
Update in
-
Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation.Cochrane Database Syst Rev. 2022 Nov 25;11(11):CD011335. doi: 10.1002/14651858.CD011335.pub3. Cochrane Database Syst Rev. 2022. PMID: 36427235 Free PMC article.
Abstract
Background: Cognitive deficits are common in people who have received cranial irradiation and have a serious impact on daily functioning and quality of life. The benefit of pharmacological and non-pharmacological treatment of cognitive deficits in this population is unclear.
Objectives: To assess the effectiveness of interventions for preventing or ameliorating cognitive deficits in adult patients treated with cranial irradiation.
Search methods: In August 2014. we searched the Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and PsycINFO and checked the reference lists of included studies. We also searched for ongoing trials via ClinicalTrials.gov, the Physicians Data Query and the Meta Register of Controlled Trials.
Selection criteria: We included randomised controlled trials (RCTs) that evaluated pharmacological or non-pharmacological interventions in cranial irradiated adults, with objective cognitive functioning as a primary or secondary outcome measure.
Data collection and analysis: Two review authors (JD, KZ) independently extracted data from selected studies and carried out a 'Risk of bias' assessment. Cognitive function, fatigue and mood outcomes were reported. No data were pooled.
Main results: Sixteen studies were identified for possible inclusion in the review, six of which were included. Three studies investigated prevention and three studies investigated amelioration. Due to differences between studies in the interventions being evaluated, a meta-analysis was not possible. Two studies investigated a pharmacological intervention for the prevention of cognitive deficits; memantine compared with placebo, and d-threo-methylphenidate HCL compared with placebo. In the first study the primary cognitive outcome of memory at six months did not reach significance, but there was significant improvement in overall cognitive function compared to placebo, with similar adverse events across groups. The second study found no statistically significant difference between arms, with few adverse events. The third study investigated a rehabilitation program for the prevention of cognitive deficits but did not carry out a statistical comparison of cognitive performance between groups.Three studies investigated the use of a pharmacological intervention for the treatment of cognitive deficits; methylphenidate compared with modafinil, two different doses of modafinil, and donepezil compared with placebo. The first study found improvements in cognitive function in both the methylphenidate and modafinil arms; few adverse events were reported. The second study combined treatment arms and found improvements across all cognitive tests, however, a number of adverse events were reported. Both studies were limited by a small sample size. The third study did not find an improvement in the primary cognitive outcome of overall performance, but did find improvement in an individual test of memory, compared to placebo; adverse events were not reported. No non-pharmacological studies for the amelioration of cognitive deficits were eligible. There were a number of limitations across studies but few without high risks of bias.
Authors' conclusions: There is supportive evidence that memantine may help prevent cognitive deficits for adults with brain metastases receiving cranial irradiation. There is supportive evidence that donepezil may have a role in treating cognitive deficits in adults with primary or metastatic brain tumours who have been treated with cranial irradiation. Patient withdrawal affected the statistical power of both studies. Further research that tries to minimise the withdrawal of consent, and subsequently reduce the requirement for imputation procedures, may offer a higher quality of evidence.There is no strong evidence to support any non-pharmacological interventions (medical or cognitive/behavioural) in the prevention or amelioration of cognitive deficits. Non-randomised studies appear promising but are as yet to be conclusive via translation into high quality evidence. Further research is required.
Conflict of interest statement
Julia Day: nothing to declare Karolis Zienius: nothing to declare Martin Taphoorn: nothing to declare Jing Li: nothing to declare Karin Gehring: nothing to declare David Grosshans: nothing to declare Robin Grant: nothing to declare Paul Brown: nothing to declare
Figures
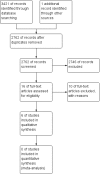



















References
References to studies included in this review
-
- Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole‐brain radiotherapy: a randomized, double‐blind, placebo‐controlled trial. Neuro‐Oncology 2013;15(10):1429‐37. [DOI: 10.1093/neuonc/not114] - DOI - PMC - PubMed
-
- Butler JM Jr, Case LD, Atkins J, Frizzell B, Sanders G, Griffin P, et al. A phase III, double‐blind, placebo‐controlled prospective randomised clinical trial of d‐threo‐methylphenidate HCl in brain tumour patients receiving radiation therapy. Internation Journal of Radiation Oncology *Biology *Physics 2007;69(5):1496‐501. [DOI: 10.1016/j.ijrobp.2007.05.076] - DOI - PubMed
-
- Gehring K, Patwardhan SY, Collins R, Groves MD, Etzel CJ, Meyers CA, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. Journal of Neuro‐Oncology 2012;107(1):165‐74. [DOI: 10.1007/s11060-011-0723-1] - DOI - PubMed
-
- Kaleita TA, Wellisch DK, Graham CA, Steh B, Nghiemphu, Ford JM, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors. Journal of Clinical Oncology. 2006; Vol. 24, issue 18S:1503.
-
- Locke DEC, Cerhan JH, Wu W, Malec JF, Clark MM, Rummans TA, et al. Cognitive rehabilitation and problem‐solving to improve quality of life of patients with primary brain tumours: a pilot study. Journal of Supportive Oncology 2008;6(8):383‐91. - PubMed
References to studies excluded from this review
-
- Hulshof MCCM, Stark NM, Kleij A, Sminia P, Smeding HMM, Gonzalez DG. Hyperbaric oxygen therapy for cognitive disorders after irradiation of the brain [Hyperbare oxygenierung bei patienten mit kognitiven störungen nach hirnbestrahlung]. Strahlentherapie und Onkologie 2002;178(4):192‐8. [DOI: 10.1007/s00066-002-0916-9] - DOI - PubMed
References to studies awaiting assessment
References to ongoing studies
-
- Umphrey ABP. Armodafinil in reducing cancer‐related fatigue in patients with high grade glioma. ClinicalTrials.gov2013; Vol. NCT01781468.
Additional references
-
- Abrey LE, DeAngelis LM, Yahalom J. Long‐term survival in primary CNS lymphoma. Journal of Clinical Oncology 1998;16(3):859‐63. - PubMed
-
- Anscher MS, Swift PS, Gaspar LE, Marks LB. Radiation injury of the brain and spinal cord. In: Wilkins RH, Rengachary SS editor(s). Neurosurgery. New York: McGraw‐Hill, 1996:1921‐36.
-
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test‐revised: normative data and analysis of inter‐form and test‐retest reliability. Clinical Neuropsychologist 1998;12(1):43‐55.
-
- Benton AL, Hamsher KS. Multilingual Aphasia Examination. Iowa City, IA, AJA Associates1989.
-
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clinic Proceedings 2000;75(7):711‐21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

