Altered myogenic vasoconstriction and regulation of whole kidney blood flow in the ASIC2 knockout mouse
- PMID: 25520010
- PMCID: PMC4329487
- DOI: 10.1152/ajprenal.00572.2014
Altered myogenic vasoconstriction and regulation of whole kidney blood flow in the ASIC2 knockout mouse
Abstract
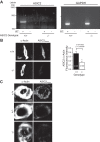
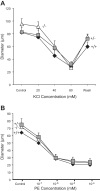
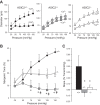
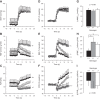
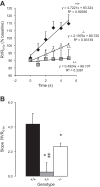
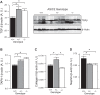
Previous studies from our laboratory have suggested that degenerin proteins contribute to myogenic constriction, a mechanism of blood flow regulation and protection against pressure-dependent organ injury, in renal vessels. The goal of the present study was to determine the importance of one family member, acid-sensing ion channel 2 (ASIC2), in myogenic constriction of renal interlobar arteries, myogenic regulation of whole kidney blood flow, renal injury, and blood pressure using ASIC2(+/+), ASIC2(+/-), and ASIC2(-/-) mice. Myogenic constriction in renal interlobar arteries was impaired in ASIC2(+/-) and ASIC2(-/-) mice, whereas constriction to KCl/phenylephrine was unchanged. Correction of whole kidney renal vascular resistance (RVR) during the first 5 s after a 10- to 20-mmHg step increase in perfusion pressure, a timeframe associated with myogenic-mediated correction of RVR, was slowed (4.2 ± 0.9, 0.3 ± 0.7, and 2.4 ± 0.3 resistance units/s in ASIC2(+/+), ASIC2(+/-), and ASIC2(-/-) mice). Although modest reductions in function were observed in ASIC2(-/-) mice, greater reductions were observed in ASIC2(+/-) mice, which may be explained by protein-protein interactions of ASIC2 with other degenerins. Isolated glomeruli from ASIC2(+/-) and ASIC2(-/-) mice had modest alterations in the expression of inflammation and injury markers (transforming growth factor-β, mouse anti-target of antiproliferative antibody-1, and nephrin), whereas ASIC2(+/-) mice had an increase in the remodeling marker collagen type III. Consistent with a more severe loss of function, mean arterial pressure was increased in ASIC2(+/-) mice (131 ± 3 mmHg) but not in ASIC2(-/-) mice (122 ± 3 vs. 117 ± 2 mmHg in ASIC2(+/+) mice). These results suggest that ASIC2 contributes to transduction of the renal myogenic response and are consistent with the protective role of myogenic constriction against renal injury and hypertension.
Keywords: acid-sensing ion channel 2; degenerin; pressure-induced constriction; renal injury.
Copyright © 2015 the American Physiological Society.
Figures
Similar articles
-
βENaC and ASIC2 associate in VSMCs to mediate pressure-induced constriction in the renal afferent arteriole.Am J Physiol Renal Physiol. 2022 May 1;322(5):F498-F511. doi: 10.1152/ajprenal.00003.2022. Epub 2022 Mar 14. Am J Physiol Renal Physiol. 2022. PMID: 35285274 Free PMC article.
-
Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice.Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1793-803. doi: 10.1152/ajpheart.01380.2007. Epub 2008 Feb 22. Am J Physiol Heart Circ Physiol. 2008. PMID: 18296560
-
Vascular smooth muscle function of renal glomerular and interlobar arteries predicts renal damage in rats.Am J Physiol Renal Physiol. 2012 Oct 15;303(8):F1187-95. doi: 10.1152/ajprenal.00653.2011. Epub 2012 Jul 11. Am J Physiol Renal Physiol. 2012. PMID: 22791345
-
Renal autoregulation in health and disease.Physiol Rev. 2015 Apr;95(2):405-511. doi: 10.1152/physrev.00042.2012. Physiol Rev. 2015. PMID: 25834230 Free PMC article. Review.
-
A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle.Physiology (Bethesda). 2008 Feb;23:23-31. doi: 10.1152/physiol.00034.2007. Physiology (Bethesda). 2008. PMID: 18268362 Review.
Cited by
-
The Novel ASIC2 Locus is Associated with Severe Gingival Inflammation.JDR Clin Trans Res. 2016 Jul;1(2):163-170. doi: 10.1177/2380084416645290. Epub 2016 Apr 20. JDR Clin Trans Res. 2016. PMID: 28459102 Free PMC article.
-
Loss of acid-sensing ion channel 2 enhances pulmonary vascular resistance and hypoxic pulmonary hypertension.J Appl Physiol (1985). 2019 Aug 1;127(2):393-407. doi: 10.1152/japplphysiol.00894.2018. Epub 2019 Jun 6. J Appl Physiol (1985). 2019. PMID: 31169471 Free PMC article.
-
βENaC and ASIC2 associate in VSMCs to mediate pressure-induced constriction in the renal afferent arteriole.Am J Physiol Renal Physiol. 2022 May 1;322(5):F498-F511. doi: 10.1152/ajprenal.00003.2022. Epub 2022 Mar 14. Am J Physiol Renal Physiol. 2022. PMID: 35285274 Free PMC article.
-
Vascular mechanotransduction.Physiol Rev. 2023 Apr 1;103(2):1247-1421. doi: 10.1152/physrev.00053.2021. Epub 2023 Jan 5. Physiol Rev. 2023. PMID: 36603156 Free PMC article. Review.
-
Interleukin-17 induces hypertension but does not impair cerebrovascular function in pregnant rats.Pregnancy Hypertens. 2021 Jun;24:50-57. doi: 10.1016/j.preghy.2021.02.009. Epub 2021 Feb 23. Pregnancy Hypertens. 2021. PMID: 33677419 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

