Conformation transitions of eukaryotic polyribosomes during multi-round translation
- PMID: 25520190
- PMCID: PMC4288168
- DOI: 10.1093/nar/gku1270
Conformation transitions of eukaryotic polyribosomes during multi-round translation
Abstract
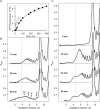
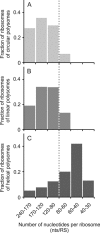
Using sedimentation and cryo electron tomography techniques, the conformations of eukaryotic polyribosomes formed in a long-term cell-free translation system were analyzed over all the active system lifetime (20-30 translation rounds during 6-8 h in wheat germ extract at 25°C). Three distinct types of the conformations were observed: (i) circular polyribosomes, varying from ring-shaped forms to circles collapsed into double rows, (ii) linear polyribosomes, tending to acquire planar zigzag-like forms and (iii) densely packed 3D helices. At the start, during the first two rounds of translation mostly the circular (ring-shaped and double-row) polyribosomes and the linear (free-shaped and zigzag-like) polyribosomes were formed ('juvenile phase'). The progressive loading of the polyribosomes with translating ribosomes induced the opening of the circular polyribosomes and the transformation of a major part of the linear polyribosomes into the dense 3D helices ('transitional phase'). After 2 h from the beginning (about 8-10 rounds of translation) this compact form of polyribosomes became predominant, whereas the circular and linear polyribosome fractions together contained less than half of polysomal ribosomes ('steady-state phase'). The latter proportions did not change for several hours. Functional tests showed a reduced translational activity in the fraction of the 3D helical polyribosomes.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
-
- Mathias A.P., Williamson R., Huxley H.E., Page S. Occurrence and function of polysomes in rabbit reticulocytes. J. Mol. Biol. 1964;9:154–167. - PubMed
-
- Shelton E., Kuff E.L. Substructure and configuration of ribosomes isolated from mammalian cells. J. Mol. Biol. 1966;22:23–31.
-
- Christensen A.K., Kahn L.E., Bourne C.M. Circular polysomes predominate on the rough endoplasmic reticulum of somatotropes and mammotropes in the rat anterior pituitary. Am. J. Anat. 1987;178:1–10. - PubMed
-
- Yoshida T., Wakiyama M., Yazaki K., Miura K. Transmission electron and atomic force microscopic observation of polysomes on carbon-coated grids prepared by surface spreading. J. Electron Microsc. (Japan) 1997;46:503–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

