A cluster-randomized controlled trial of a multicomponent intervention protocol for pneumonia prevention among nursing home elders
- PMID: 25520333
- PMCID: PMC4415071
- DOI: 10.1093/cid/ciu935
A cluster-randomized controlled trial of a multicomponent intervention protocol for pneumonia prevention among nursing home elders
Abstract
Background: Pneumonia remains an important public health problem among elderly nursing home residents. This clinical trial sought to determine if a multicomponent intervention protocol, including manual tooth/gum brushing plus 0.12% chlorhexidine oral rinse, twice per day, plus upright positioning during feeding, could reduce the incidence of radiographically documented pneumonia among nursing home residents, compared with usual care.
Methods: This cluster-randomized clinical trial was conducted in 36 nursing homes in Connecticut. Eligible residents >65 years with at least 1 of 2 modifiable risk factors for pneumonia (ie, impaired oral hygiene, swallowing difficulty) were enrolled. Nursing homes were randomized to the multicomponent intervention protocol or usual care. Participants were followed for up to 2.5 years for development of the primary outcome, a radiographically documented pneumonia, and secondary outcome, a lower respiratory tract infection (LRTI) without radiographic documentation.
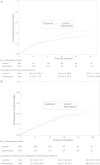
Results: A total of 834 participants were enrolled: 434 to intervention and 400 to usual care. The trial was terminated for futility. The number of participants in the intervention vs control arms with first pneumonia was 119 (27.4%) vs 94 (23.5%), respectively, and with first LRTI, 125 (28.8%) vs 100 (25.0%), respectively. In a multivariable Cox regression model, the hazard ratio in the intervention vs control arms, respectively, was 1.12 (95% confidence interval [CI], .84-1.50; P = .44) for first pneumonia and 1.07 (95% CI, .79-1.46, P = .65) for first LRTI.
Conclusions: The multicomponent intervention protocol did not significantly reduce the incidence of first radiographically confirmed pneumonia or LRTI compared with usual care in nursing home residents.
Clinical trials registration: NCT00975780.
Keywords: chlorhexidine; nursing homes; oral care; pneumonia; prevention.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Editorial commentary: Preventing aspiration pneumonia in high-risk nursing home residents: role of chlorhexidine-based oral care questioned again.Clin Infect Dis. 2015 Mar 15;60(6):858-9. doi: 10.1093/cid/ciu941. Epub 2014 Dec 16. Clin Infect Dis. 2015. PMID: 25520334 No abstract available.
References
-
- World Health Organization. Health statistics and information systems. Available at: http://www.who.int/healthinfo/global_burden_disease. Accessed 2 December 2014.
-
- High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:149–71. - PubMed
-
- Quagliarello V, Ginter S, Han L, Van Ness P, Allore H, Tinetti M. Modifiable risk factors for nursing home-acquired pneumonia. Clin Infect Dis. 2005;40:1–6. - PubMed
-
- Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Amer Geriatr Soc. 2003;51:1018–22. - PubMed
-
- Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Amer Geriatr Soc. 2002;50:430–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

