Modified vaccinia virus Ankara-infected dendritic cells present CD4+ T-cell epitopes by endogenous major histocompatibility complex class II presentation pathways
- PMID: 25520512
- PMCID: PMC4325755
- DOI: 10.1128/JVI.03244-14
Modified vaccinia virus Ankara-infected dendritic cells present CD4+ T-cell epitopes by endogenous major histocompatibility complex class II presentation pathways
Abstract
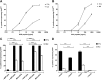
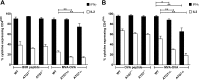
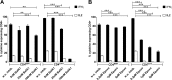
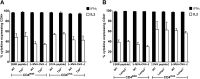
CD4(+) T lymphocytes play a central role in the immune system and mediate their function after recognition of their respective antigens presented on major histocompatibility complex II (MHCII) molecules on antigen-presenting cells (APCs). Conventionally, phagocytosed antigens are loaded on MHCII for stimulation of CD4(+) T cells. Certain epitopes, however, can be processed directly from intracellular antigens and are presented on MHCII (endogenous MHCII presentation). Here we characterized the MHCII antigen presentation pathways that are possibly involved in the immune response upon vaccination with modified vaccinia virus Ankara (MVA), a promising live viral vaccine vector. We established CD4(+) T-cell lines specific for MVA-derived epitopes as tools for in vitro analysis of MHCII antigen processing and presentation in MVA-infected APCs. We provide evidence that infected APCs are able to directly transfer endogenous viral proteins into the MHCII pathway to efficiently activate CD4(+) T cells. By using knockout mice and chemical inhibitory compounds, we further elucidated the molecular basis, showing that among the various subcellular pathways investigated, proteasomes and autophagy are key players in the endogenous MHCII presentation during MVA infection. Interestingly, although proteasomal processing plays an important role, neither TAP nor LAMP-2 was found to be involved in the peptide transport. Defining the molecular mechanism of MHCII presentation during MVA infection provides a basis for improving MVA-based vaccination strategies by aiming for enhanced CD4(+) T-cell activation by directing antigens into the responsible pathways.
Importance: This work contributes significantly to our understanding of the immunogenic properties of pathogens by deciphering antigen processing pathways contributing to efficient activation of antigen-specific CD4(+) T cells. We identified autophagosome formation, proteasomal activity, and lysosomal integrity as being crucial for endogenous CD4(+) T-cell activation. Since poxvirus vectors such as MVA are already used in clinical trials as recombinant vaccines, the data provide important information for the future design of optimized poxviral vaccines for the study of advanced immunotherapy options.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

