The extraocular muscle stem cell niche is resistant to ageing and disease
- PMID: 25520657
- PMCID: PMC4249457
- DOI: 10.3389/fnagi.2014.00328
The extraocular muscle stem cell niche is resistant to ageing and disease
Abstract
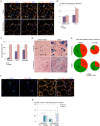
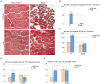
Specific muscles are spared in many degenerative myopathies. Most notably, the extraocular muscles (EOMs) do not show clinical signs of late stage myopathies including the accumulation of fibrosis and fat. It has been proposed that an altered stem cell niche underlies the resistance of EOMs in these pathologies, however, to date, no reports have provided a detailed characterization of the EOM stem cell niche. PW1/Peg3 is expressed in progenitor cells in all adult tissues including satellite cells and a subset of interstitial non-satellite cell progenitors in muscle. These PW1-positive interstitial cells (PICs) include a fibroadipogenic progenitor population (FAP) that give rise to fat and fibrosis in late stage myopathies. PICs/FAPs are mobilized following injury and FAPs exert a promyogenic role upon myoblasts in vitro but require the presence of a minimal population of satellite cells in vivo. We and others recently described that FAPs express promyogenic factors while satellite cells express antimyogenic factors suggesting that PICs/FAPs act as support niche cells in skeletal muscle through paracrine interactions. We analyzed the EOM stem cell niche in young adult and aged wild-type mice and found that the balance between PICs and satellite cells within the EOM stem cell niche is maintained throughout life. Moreover, in the adult mdx mouse model for Duchenne muscular dystrophy (DMD), the EOM stem cell niche is unperturbed compared to normal mice, in contrast to Tibialis Anterior (TA) muscle, which displays signs of ongoing degeneration/regeneration. Regenerating mdx TA shows increased levels of both PICs and satellite cells, comparable to normal unaffected EOMs. We propose that the increase in PICs that we observe in normal EOMs contributes to preserving the integrity of the myofibers and satellite cells. Our data suggest that molecular cues regulating muscle regeneration are intrinsic properties of EOMs.
Keywords: Duchenne muscular dystrophy; PICs; extraocular muscles; muscle stem cell niche; satellite cells.
Figures
References
-
- Besson V., Smeriglio P., Wegener A., Relaix F., Nait Oumesmar B., Sassoon D. A., et al. . (2011). PW1 gene/paternally expressed gene 3 (PW1/Peg3) identifies multiple adult stem and progenitor cell populations. Proc. Natl. Acad. Sci. U S A 108, 11470–11475. 10.1073/pnas.1103873108 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

