Stem cell transplantation as a dynamical system: are clinical outcomes deterministic?
- PMID: 25520720
- PMCID: PMC4253954
- DOI: 10.3389/fimmu.2014.00613
Stem cell transplantation as a dynamical system: are clinical outcomes deterministic?
Abstract
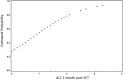
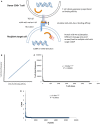
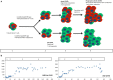
Outcomes in stem cell transplantation (SCT) are modeled using probability theory. However, the clinical course following SCT appears to demonstrate many characteristics of dynamical systems, especially when outcomes are considered in the context of immune reconstitution. Dynamical systems tend to evolve over time according to mathematically determined rules. Characteristically, the future states of the system are predicated on the states preceding them, and there is sensitivity to initial conditions. In SCT, the interaction between donor T cells and the recipient may be considered as such a system in which, graft source, conditioning, and early immunosuppression profoundly influence immune reconstitution over time. This eventually determines clinical outcomes, either the emergence of tolerance or the development of graft versus host disease. In this paper, parallels between SCT and dynamical systems are explored and a conceptual framework for developing mathematical models to understand disparate transplant outcomes is proposed.
Keywords: T cell repertoire; dynamical system; graft versus host disease; logistic function; stem cell transplantation.
Figures




References
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood (1990) 75:555–62. - PubMed
-
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood (1995) 86:2041–50. - PubMed
-
- Valcárcel D, Sierra J, Wang T, Kan F, Gupta V, Hale GA, et al. One-antigen mismatched related versus HLA-matched unrelated donor hematopoietic stem cell transplantation in adults with acute leukemia: Center for International Blood and Marrow Transplant Research results in the era of molecular HLA typing. Biol Blood Marrow Transplant (2011) 17:640–8.10.1016/j.bbmt.2010.07.022 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

