Methylation-associated silencing of MicroRNA-335 contributes tumor cell invasion and migration by interacting with RASA1 in gastric cancer
- PMID: 25520857
- PMCID: PMC4266701
Methylation-associated silencing of MicroRNA-335 contributes tumor cell invasion and migration by interacting with RASA1 in gastric cancer
Abstract
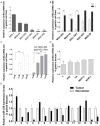
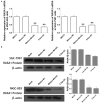
MicroRNAs (miRNAs) are small non-coding RNAs that function as endogenous silencers of target genes, previous studies have shown that miR-335 play an important role in suppressing metastasis and migration in human cancer including gastric cancer (GC). However, the mechanisms which result in aberrant expression of miR-335 in GC are still unknown. Recent studies have shown that the silencing of some miRNAs is associated with DNA hypermethylation. In this study, we find the promoter of miR-335 we embedded in CpG island by accessing to bioinformatics data and the low expression of miR-335 in 5 gastric cell lines can be restored by 5-aza-2'-deoxycytidine (5-Aza-dC) treatment. So we postulated that the miR-335 genes undergo epigenetic inactivation in GC. Subsequently, in GC cells and tissues, we performed quantitative real-time PCR (RTQ-PCR) to assess the expression of miR-335, and methylation-specific PCR (MSP) and bisulfite sequence-PCR (BSP) to evaluate the DNA methylation status in the CpG islands upstream of MiR-335. The result showed that the expression of miR-335 was significantly reduce in gastric cancer cell lines and tumor tissues compared to matched normal gastric tissues, and cell lines, and which is inverse correlation with DNA hypermethylation of miR-335 both in GC cells lines and tissues, but not in normal tissues. In addition, we found that the lower miR-335 expression induced by abnormal methylation may be mainly involved in gastric cell invasion and metastasis in GC tissues. No statistical significance was found about miR-335 expression and methylation level between healthy individuals with and without H. pylori (HP) infection. Finally, we carry out miRNA transfection, RTQ-PCR and western blot assay to find the RAS p21 protein activator (GTPase activating protein) 1 (RASA1) may be the possible target genes which lead to the gastric cell invasion and metastasis, furthermore, the re-expression of endogenous miR-335 by 5-Aza-dC treatment can exert effects similar to exogenous miRNAs transfection. Taken together, our results suggest that miR-335 may be silenced by promoter hypermethylation and play important roles in gastric cell invasion and metastasis through its target genes, such as RASA1. Its methylation level might be a predictive epigenetic marker of GC and remodeling on the expression by demethylation can provided a potential therapeutic strategy.
Keywords: Cancer metastasis; MiR-335; RASA1; cancer invasion; gastric carcinoma; methylation.
Figures




References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Welch DR, Rinker-Schaeffer CW. What defines a useful marker of metastasis in human cancer? J Natl Cancer Inst. 1999;91:1351–1353. - PubMed
-
- Hoon DS, Kitago M, Kim J, Mori T, Piris A, Szyfelbein K, Mihm MC Jr, Nathanson SD, Padera TP, Chambers AF, Vantyghem SA, MacDonald IC, Shivers SC, Alsarraj M, Reintgen DS, Passlick B, Sienel W, Pantel K. Molecular mechanisms of metastasis. Cancer Metastasis Rev. 2006;25:203–220. - PubMed
-
- Aigner A. MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl) 2011;89:445–457. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
