Establishment of genetically diverse patient-derived xenografts of colorectal cancer
- PMID: 25520871
- PMCID: PMC4266715
Establishment of genetically diverse patient-derived xenografts of colorectal cancer
Abstract
Preclinical compounds tested in animal models often show limited efficacy when transitioned into human clinical trials. As a result, many patients are stratified into treatment regimens that have little impact on their disease. In order to create preclinical models that can more accurately predict tumor responses, we established patient-derived xenograft (PDX) models of colorectal cancer (CRC). Surgically resected tumor specimens from colorectal cancer patients were implanted subcutaneously into athymic nude mice. Following successful establishment, fourteen models underwent further evaluation to determine whether these models exhibit heterogeneity, both at the cellular and genetic level. Histological review revealed properties not found in CRC cell lines, most notably in overall architecture (predominantly columnar epithelium with evidence of gland formation) and the presence of mucin-producing cells. Custom CRC gene panels identified somatic driver mutations in each model, and therapeutic efficacy studies in tumor-bearing mice were designed to determine how models with known mutations respond to PI3K, mTOR, or MAPK inhibitors. Interestingly, MAPK pathway inhibition drove tumor responses across most models tested. Noteworthy, the MAPK inhibitor PD0325901 alone did not significantly mediate tumor response in the context of a KRAS(G12D) model, and improved tumor responses resulted when combined with mTOR inhibition. As a result, these genetically diverse models represent a valuable resource for preclinical efficacy and drug discovery studies.
Keywords: AZD8055; BEZ235; PD0325901; Targeted therapies; colorectal cancer; patient-derived xenograft; translational models.
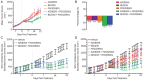
Figures




References
-
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. - PubMed
-
- Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–3354. discussion 3354. - PubMed
-
- Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, Yung R, Parmigiani G, Dorsch M, Peacock CD, Watkins DN. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–3373. - PMC - PubMed
-
- Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
